 Found 73 hits with Last Name = 'hanna' and Initial = 'c'
Found 73 hits with Last Name = 'hanna' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026212
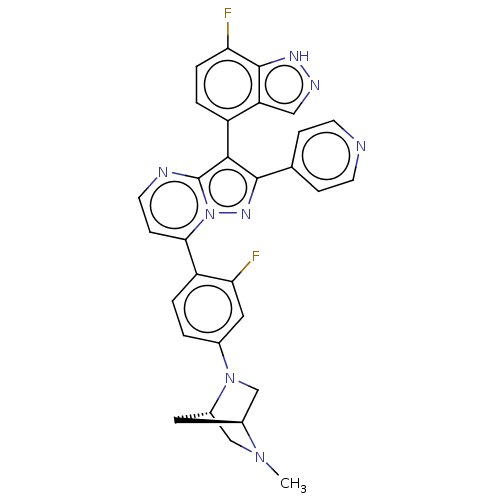
(CHEMBL1276185)Show SMILES [H][C@]12CN(c3ccc(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:36.42,1.0,(5.96,-12.33,;4.87,-13.43,;4.88,-14.97,;3.54,-15.75,;3.55,-17.28,;4.89,-18.05,;4.89,-19.59,;3.56,-20.36,;2.23,-19.6,;.9,-20.38,;2.22,-18.06,;3.57,-21.9,;2.24,-22.67,;2.24,-24.21,;3.57,-24.98,;4.91,-24.21,;6.38,-24.69,;7.29,-23.44,;6.38,-22.18,;4.91,-22.66,;8.83,-23.43,;9.6,-24.77,;11.14,-24.77,;11.91,-23.43,;11.13,-22.09,;9.6,-22.1,;6.86,-26.15,;8.36,-26.47,;8.84,-27.93,;7.81,-29.09,;8.29,-30.55,;6.3,-28.76,;5.05,-29.66,;3.81,-28.75,;4.3,-27.29,;5.83,-27.3,;2.21,-14.99,;.66,-14.98,;2.2,-13.45,;3.53,-12.67,;3.53,-11.12,;3.29,-13.89,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026207
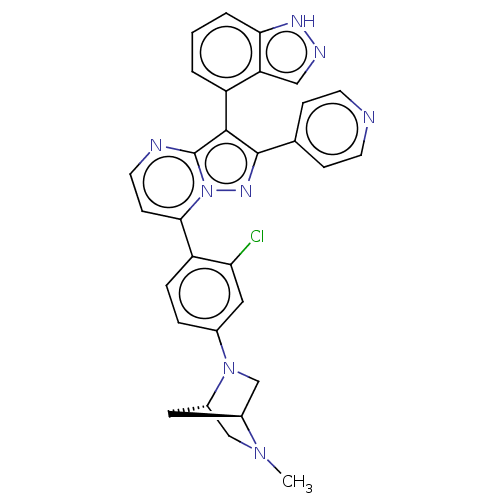
(CHEMBL1276170)Show SMILES [H][C@]12CN(c3ccc(c(Cl)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:35.41,1.0,(38.98,4.96,;37.9,3.86,;37.9,2.32,;36.57,1.54,;36.58,.01,;37.92,-.76,;37.92,-2.3,;36.58,-3.07,;35.25,-2.31,;33.92,-3.09,;35.24,-.77,;36.59,-4.61,;35.26,-5.38,;35.26,-6.93,;36.6,-7.7,;37.93,-6.92,;39.41,-7.4,;40.32,-6.15,;39.41,-4.89,;37.93,-5.37,;41.86,-6.14,;42.63,-7.48,;44.17,-7.48,;44.94,-6.15,;44.16,-4.81,;42.62,-4.81,;39.88,-8.87,;41.39,-9.18,;41.87,-10.64,;40.83,-11.8,;39.32,-11.48,;38.08,-12.37,;36.84,-11.46,;37.32,-10.01,;38.86,-10.01,;35.24,2.3,;33.69,2.3,;35.22,3.84,;36.56,4.62,;36.55,6.17,;36.32,3.4,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330925
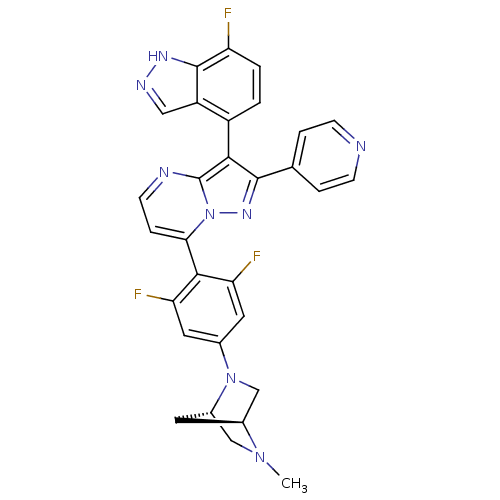
(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c2[nH]ncc12 |r,wU:3.3,5.4,(16.85,-11.72,;16.86,-13.27,;15.53,-14.05,;15.54,-15.59,;16.62,-14.49,;18.2,-14.03,;18.21,-15.57,;16.87,-16.35,;16.88,-17.88,;15.54,-18.66,;15.55,-20.2,;14.22,-20.98,;16.89,-20.96,;18.22,-20.19,;19.56,-20.96,;18.22,-18.65,;16.9,-22.5,;15.57,-23.27,;15.57,-24.82,;16.9,-25.59,;18.24,-24.81,;19.71,-25.29,;20.62,-24.04,;19.71,-22.78,;18.23,-23.26,;22.16,-24.03,;22.93,-25.37,;24.47,-25.37,;25.24,-24.04,;24.46,-22.7,;22.92,-22.7,;20.19,-26.76,;21.69,-27.07,;22.17,-28.53,;21.14,-29.69,;21.62,-31.15,;19.63,-29.37,;18.38,-30.26,;17.14,-29.36,;17.62,-27.9,;19.16,-27.9,)| Show InChI InChI=1S/C30H23F3N8/c1-39-14-19-10-18(39)15-40(19)17-11-23(32)27(24(33)12-17)25-6-9-35-30-26(20-2-3-22(31)29-21(20)13-36-37-29)28(38-41(25)30)16-4-7-34-8-5-16/h2-9,11-13,18-19H,10,14-15H2,1H3,(H,36,37)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330905
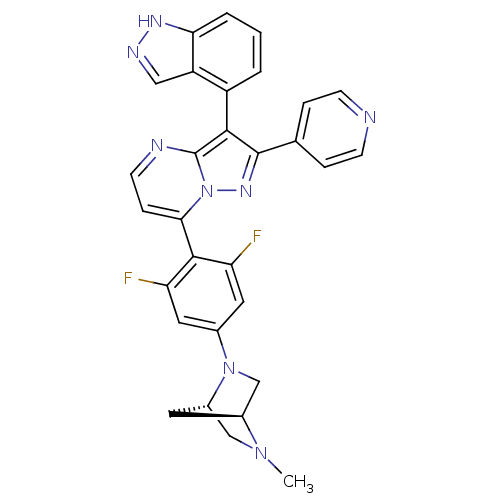
(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(24.13,-33.61,;24.13,-35.15,;22.8,-35.94,;22.81,-37.47,;23.89,-36.37,;25.47,-35.92,;25.48,-37.46,;24.14,-38.23,;24.15,-39.77,;22.82,-40.55,;22.83,-42.09,;21.5,-42.86,;24.16,-42.85,;25.5,-42.07,;26.83,-42.84,;25.49,-40.53,;24.17,-44.38,;22.84,-45.15,;22.84,-46.7,;24.17,-47.47,;25.51,-46.7,;26.98,-47.18,;27.89,-45.92,;26.98,-44.67,;25.51,-45.15,;29.43,-45.92,;30.2,-47.26,;31.74,-47.26,;32.51,-45.92,;31.73,-44.58,;30.2,-44.59,;27.46,-48.64,;28.96,-48.96,;29.44,-50.42,;28.41,-51.57,;26.9,-51.25,;25.65,-52.15,;24.41,-51.24,;24.9,-49.78,;26.43,-49.79,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-11-19(38)16-39(20)18-12-23(31)28(24(32)13-18)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,19-20H,11,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330924
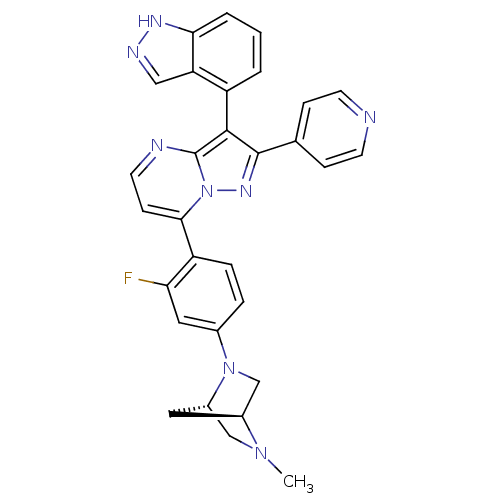
(7-(2-fluoro-4-((1S,4S)-5-methyl-2,5-diazabicyclo[2...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(25.08,5.67,;25.09,4.13,;23.75,3.34,;23.77,1.8,;24.85,2.9,;26.43,3.36,;26.44,1.82,;25.1,1.04,;25.11,-.49,;26.45,-1.26,;26.45,-2.8,;25.12,-3.57,;23.78,-2.81,;22.45,-3.59,;23.77,-1.27,;25.13,-5.11,;23.8,-5.88,;23.79,-7.42,;25.13,-8.19,;26.46,-7.42,;27.94,-7.9,;28.85,-6.65,;27.94,-5.39,;26.46,-5.87,;30.39,-6.64,;31.16,-7.98,;32.7,-7.98,;33.47,-6.64,;32.69,-5.3,;31.15,-5.31,;28.41,-9.36,;29.92,-9.68,;30.4,-11.14,;29.36,-12.29,;27.85,-11.97,;26.61,-12.87,;25.37,-11.96,;25.85,-10.5,;27.39,-10.51,)| Show InChI InChI=1S/C30H25FN8/c1-37-16-21-13-20(37)17-38(21)19-5-6-23(25(31)14-19)27-9-12-33-30-28(22-3-2-4-26-24(22)15-34-35-26)29(36-39(27)30)18-7-10-32-11-8-18/h2-12,14-15,20-21H,13,16-17H2,1H3,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026206
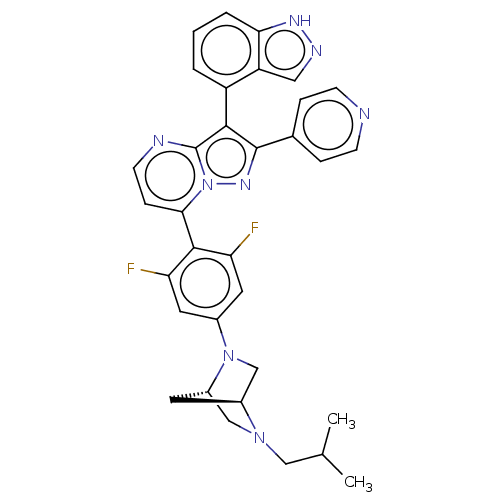
(CHEMBL1276195)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1CC(C)C)C2 |r,wU:36.42,1.0,(21.41,.41,;20.33,-.69,;20.34,-2.23,;19,-3.01,;19.01,-4.54,;17.67,-5.32,;17.69,-6.86,;16.35,-7.64,;19.02,-7.62,;20.35,-6.85,;21.69,-7.62,;20.35,-5.31,;19.03,-9.16,;17.7,-9.93,;17.7,-11.48,;19.03,-12.25,;20.37,-11.47,;21.84,-11.95,;22.75,-10.7,;21.84,-9.44,;20.36,-9.92,;24.29,-10.69,;25.06,-12.03,;26.6,-12.03,;27.37,-10.7,;26.59,-9.36,;25.06,-9.36,;22.32,-13.42,;23.82,-13.73,;24.3,-15.19,;23.27,-16.35,;21.76,-16.03,;20.51,-16.92,;19.27,-16.01,;19.75,-14.56,;21.29,-14.56,;17.67,-2.25,;16.12,-2.25,;17.66,-.71,;18.99,.07,;18.98,1.62,;20.31,2.39,;20.31,3.93,;21.65,1.63,;18.75,-1.15,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026208
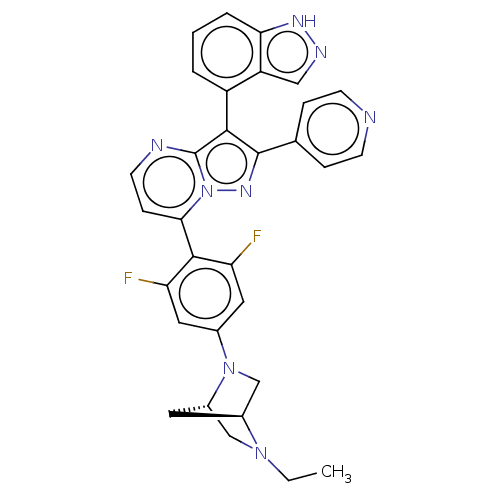
(CHEMBL1276193)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1CC)C2 |r,wU:36.42,1.0,(-2.84,3.86,;-3.92,2.75,;-3.91,1.21,;-5.25,.44,;-5.24,-1.09,;-6.58,-1.88,;-6.57,-3.41,;-7.9,-4.19,;-5.23,-4.18,;-3.9,-3.4,;-2.56,-4.17,;-3.9,-1.86,;-5.22,-5.71,;-6.55,-6.48,;-6.55,-8.03,;-5.22,-8.8,;-3.89,-8.03,;-2.41,-8.51,;-1.5,-7.25,;-2.41,-6,;-3.89,-6.48,;.04,-7.25,;.81,-8.59,;2.35,-8.59,;3.12,-7.25,;2.34,-5.91,;.8,-5.92,;-1.93,-9.97,;-.43,-10.29,;.05,-11.75,;-.98,-12.9,;-2.49,-12.58,;-3.74,-13.48,;-4.98,-12.57,;-4.5,-11.11,;-2.96,-11.12,;-6.58,1.2,;-8.13,1.2,;-6.59,2.74,;-5.26,3.52,;-5.27,5.06,;-3.94,5.84,;-5.5,2.3,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026210

(CHEMBL1276196)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C1CCC1)C2 |r,wU:36.42,1.0,(4.86,-17.8,;3.77,-18.9,;3.78,-20.44,;2.44,-21.22,;2.45,-22.75,;1.12,-23.53,;1.13,-25.07,;-.2,-25.85,;2.46,-25.83,;3.8,-25.06,;5.13,-25.82,;3.79,-23.52,;2.47,-27.37,;1.14,-28.14,;1.14,-29.68,;2.47,-30.45,;3.81,-29.68,;5.28,-30.16,;6.19,-28.9,;5.28,-27.65,;3.81,-28.13,;7.73,-28.9,;8.5,-30.24,;10.04,-30.24,;10.81,-28.9,;10.03,-27.56,;8.5,-27.57,;5.76,-31.62,;7.26,-31.94,;7.74,-33.4,;6.71,-34.55,;5.2,-34.23,;3.95,-35.13,;2.71,-34.22,;3.2,-32.76,;4.73,-32.77,;1.11,-20.46,;-.44,-20.45,;1.1,-18.92,;2.43,-18.13,;2.43,-16.59,;3.51,-15.5,;2.42,-14.42,;1.33,-15.51,;2.19,-19.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026211

(CHEMBL1276184)Show SMILES [H][C@]12CN(c3ccc(c(C)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:36.42,1.0,(-6.99,-8.62,;-8.07,-9.72,;-8.07,-11.26,;-9.4,-12.03,;-9.39,-13.57,;-8.05,-14.33,;-8.05,-15.87,;-9.39,-16.65,;-10.72,-15.89,;-12.05,-16.66,;-10.73,-14.35,;-9.38,-18.18,;-10.71,-18.95,;-10.71,-20.5,;-9.37,-21.27,;-8.04,-20.5,;-6.56,-20.98,;-5.65,-19.72,;-6.56,-18.47,;-8.04,-18.95,;-4.11,-19.72,;-3.34,-21.06,;-1.8,-21.06,;-1.03,-19.72,;-1.81,-18.38,;-3.35,-18.39,;-6.09,-22.44,;-4.59,-22.76,;-4.11,-24.22,;-5.14,-25.37,;-4.66,-26.84,;-6.65,-25.05,;-7.89,-25.95,;-9.13,-25.04,;-8.65,-23.58,;-7.11,-23.59,;-10.73,-11.27,;-12.28,-11.27,;-10.75,-9.74,;-9.41,-8.95,;-9.42,-7.41,;-9.65,-10.17,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026209

(CHEMBL1276171)Show SMILES [H][C@]12CN(c3ccc(c(Br)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:35.41,1.0,(-8.28,-15.09,;-9.36,-16.19,;-9.36,-17.73,;-10.69,-18.5,;-10.68,-20.03,;-9.35,-20.8,;-9.34,-22.34,;-10.68,-23.12,;-12.01,-22.36,;-13.34,-23.13,;-12.03,-20.82,;-10.67,-24.65,;-12.01,-25.42,;-12.01,-26.97,;-10.67,-27.74,;-9.33,-26.97,;-7.86,-27.45,;-6.94,-26.19,;-7.86,-24.94,;-9.33,-25.42,;-5.41,-26.19,;-4.64,-27.53,;-3.1,-27.53,;-2.33,-26.19,;-3.1,-24.85,;-4.64,-24.86,;-7.38,-28.91,;-5.88,-29.23,;-5.4,-30.69,;-6.43,-31.84,;-7.94,-31.52,;-9.19,-32.42,;-10.42,-31.51,;-9.94,-30.05,;-8.41,-30.06,;-12.04,-17.74,;-13.58,-17.74,;-12.05,-16.21,;-10.7,-15.42,;-10.71,-13.88,;-10.94,-16.64,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026205
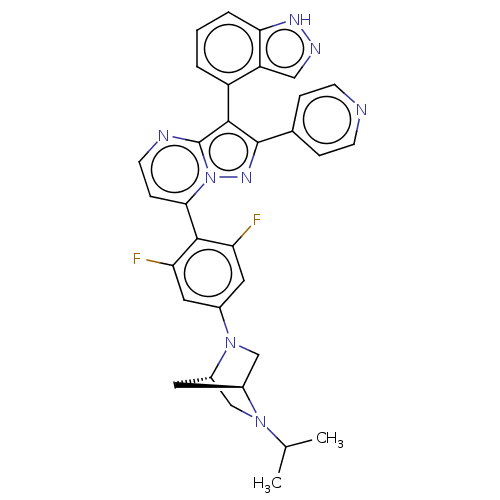
(CHEMBL1276194)Show SMILES [H][C@]12CN(c3cc(F)c(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C(C)C)C2 |r,wU:36.42,1.0,(9.92,3.92,;8.84,2.82,;8.84,1.28,;7.51,.5,;7.52,-1.03,;6.18,-1.82,;6.19,-3.35,;4.86,-4.13,;7.52,-4.11,;8.86,-3.34,;10.2,-4.11,;8.85,-1.8,;7.53,-5.65,;6.2,-6.42,;6.2,-7.97,;7.54,-8.74,;8.87,-7.97,;10.35,-8.44,;11.26,-7.19,;10.34,-5.93,;8.87,-6.41,;12.79,-7.19,;13.56,-8.52,;15.1,-8.52,;15.88,-7.19,;15.1,-5.85,;13.56,-5.85,;10.82,-9.91,;12.32,-10.22,;12.8,-11.68,;11.77,-12.84,;10.26,-12.52,;9.02,-13.41,;7.78,-12.51,;8.26,-11.05,;9.79,-11.06,;6.17,1.26,;4.63,1.26,;6.16,2.8,;7.5,3.58,;7.49,5.12,;8.82,5.9,;6.15,5.89,;7.26,2.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330923
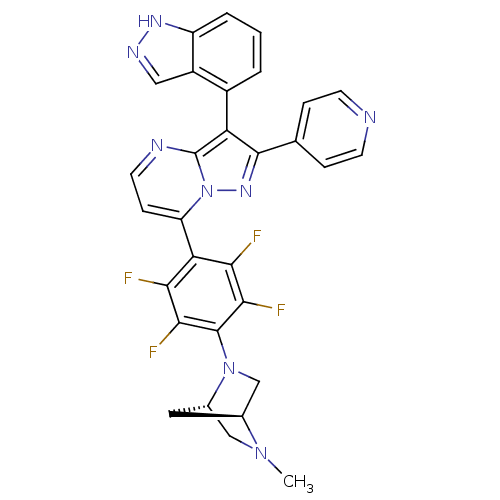
(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)-7-(2,3,5,6-te...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1c(F)c(F)c(c(F)c1F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(-7.9,5.85,;-7.89,4.3,;-9.22,3.52,;-9.21,1.98,;-8.13,3.08,;-6.55,3.54,;-6.54,2,;-7.88,1.22,;-7.87,-.31,;-6.53,-1.08,;-5.2,-.3,;-6.53,-2.62,;-5.19,-3.39,;-7.86,-3.39,;-9.19,-2.63,;-10.52,-3.41,;-9.2,-1.09,;-10.54,-.33,;-7.85,-4.93,;-9.18,-5.7,;-9.18,-7.24,;-7.85,-8.02,;-6.51,-7.24,;-5.04,-7.72,;-4.13,-6.47,;-5.04,-5.21,;-6.51,-5.69,;-2.59,-6.46,;-1.82,-7.8,;-.28,-7.8,;.49,-6.46,;-.29,-5.13,;-1.82,-5.13,;-4.56,-9.19,;-3.06,-9.5,;-2.58,-10.96,;-3.61,-12.12,;-5.12,-11.8,;-6.37,-12.69,;-7.61,-11.78,;-7.13,-10.33,;-5.59,-10.33,)| Show InChI InChI=1S/C30H22F4N8/c1-40-13-17-11-16(40)14-41(17)29-26(33)24(31)23(25(32)27(29)34)21-7-10-36-30-22(18-3-2-4-20-19(18)12-37-38-20)28(39-42(21)30)15-5-8-35-9-6-15/h2-10,12,16-17H,11,13-14H2,1H3,(H,37,38)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50311985
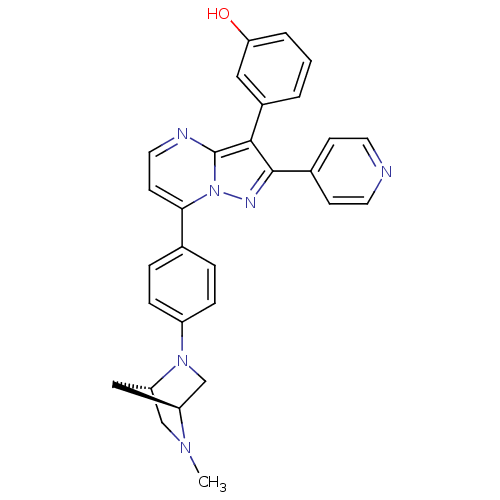
(3-(7-(4-((1S,4S)-5-methyl-2,5-diazabicyclo[2.2.1]h...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc(O)c1 |r| Show InChI InChI=1S/C29H26N6O/c1-33-17-24-16-23(33)18-34(24)22-7-5-19(6-8-22)26-11-14-31-29-27(21-3-2-4-25(36)15-21)28(32-35(26)29)20-9-12-30-13-10-20/h2-15,23-24,36H,16-18H2,1H3/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330922
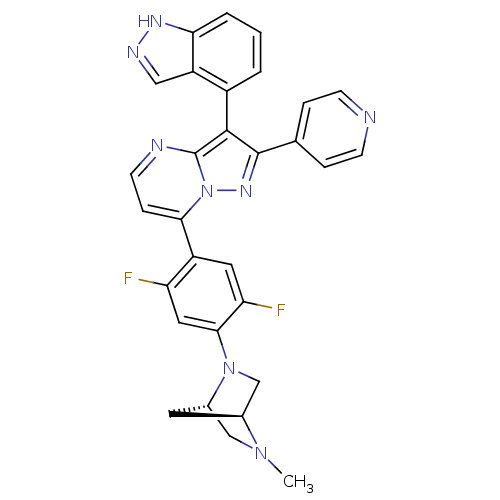
(7-(2,5-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(cc1F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(12.11,-32.8,;12.12,-34.34,;10.78,-35.12,;10.8,-36.66,;11.88,-35.56,;13.46,-35.1,;13.47,-36.64,;12.13,-37.42,;12.14,-38.95,;10.8,-39.73,;10.81,-41.27,;9.48,-42.05,;12.15,-42.03,;13.48,-41.26,;13.48,-39.72,;14.81,-38.95,;12.16,-43.57,;10.83,-44.34,;10.82,-45.89,;12.16,-46.66,;13.49,-45.88,;14.97,-46.36,;15.88,-45.11,;14.97,-43.85,;13.49,-44.33,;17.42,-45.1,;18.19,-46.44,;19.73,-46.44,;20.5,-45.11,;19.72,-43.77,;18.18,-43.77,;15.44,-47.83,;16.95,-48.14,;17.43,-49.6,;16.39,-50.76,;14.88,-50.44,;13.64,-51.33,;12.4,-50.42,;12.88,-48.97,;14.42,-48.97,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-19-11-18(38)16-39(19)27-13-23(31)21(12-24(27)32)26-7-10-34-30-28(20-3-2-4-25-22(20)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,18-19H,11,15-16H2,1H3,(H,35,36)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330921

((1S,4S)-2-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)...)Show SMILES CN1C[C@@H]2CC[C@H]1CN2c1ccc(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wD:6.6,3.9,(4.83,5.26,;4.84,3.71,;3.5,2.93,;3.51,1.39,;4.27,2.73,;5.07,1.86,;6.17,2.95,;6.18,1.41,;4.85,.63,;4.85,-.9,;6.19,-1.67,;6.2,-3.21,;4.86,-3.98,;3.53,-3.22,;2.2,-4,;3.52,-1.68,;4.87,-5.52,;3.54,-6.29,;3.54,-7.84,;4.87,-8.61,;6.21,-7.84,;7.68,-8.31,;8.59,-7.06,;7.68,-5.8,;6.21,-6.28,;10.13,-7.06,;10.9,-8.39,;12.44,-8.39,;13.22,-7.06,;12.44,-5.72,;10.9,-5.72,;8.16,-9.78,;9.66,-10.09,;10.14,-11.55,;9.11,-12.71,;7.6,-12.39,;6.35,-13.29,;5.12,-12.38,;5.6,-10.92,;7.13,-10.93,)| Show InChI InChI=1S/C31H27FN8/c1-38-17-22-6-5-21(38)18-39(22)20-7-8-24(26(32)15-20)28-11-14-34-31-29(23-3-2-4-27-25(23)16-35-36-27)30(37-40(28)31)19-9-12-33-13-10-19/h2-4,7-16,21-22H,5-6,17-18H2,1H3,(H,35,36)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330920
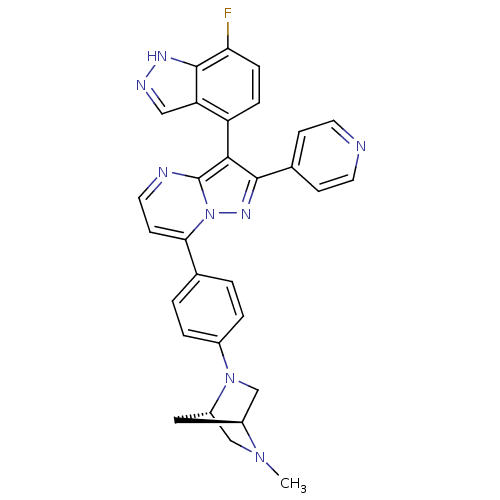
(3-(7-fluoro-1H-indazol-4-yl)-7-(4-((1S,4S)-5-methy...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c2[nH]ncc12 |r,wU:3.3,5.4,(28.5,5.92,;28.51,4.38,;27.17,3.6,;27.18,2.06,;28.27,3.16,;29.85,3.62,;29.85,2.08,;28.52,1.3,;28.53,-.23,;29.86,-1,;29.87,-2.54,;28.53,-3.31,;27.2,-2.55,;27.19,-1.01,;28.54,-4.85,;27.21,-5.62,;27.21,-7.17,;28.55,-7.94,;29.88,-7.16,;31.36,-7.64,;32.27,-6.39,;31.35,-5.13,;29.88,-5.61,;33.8,-6.38,;34.57,-7.72,;36.11,-7.72,;36.89,-6.39,;36.11,-5.05,;34.57,-5.05,;31.83,-9.11,;33.33,-9.42,;33.81,-10.88,;32.78,-12.04,;33.26,-13.5,;31.27,-11.72,;30.03,-12.61,;28.79,-11.71,;29.27,-10.25,;30.8,-10.25,)| Show InChI InChI=1S/C30H25FN8/c1-37-16-22-14-21(37)17-38(22)20-4-2-18(3-5-20)26-10-13-33-30-27(23-6-7-25(31)29-24(23)15-34-35-29)28(36-39(26)30)19-8-11-32-12-9-19/h2-13,15,21-22H,14,16-17H2,1H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330919
(3-(1H-indazol-4-yl)-7-(2-methyl-4-((1S,4S)-5-methy...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(c(C)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(14.11,6.43,;14.12,4.89,;12.79,4.1,;12.8,2.57,;13.88,3.67,;15.46,4.12,;15.47,2.58,;14.13,1.81,;14.14,.27,;15.48,-.49,;15.48,-2.03,;14.15,-2.81,;12.81,-2.05,;11.48,-2.82,;12.8,-.51,;14.16,-4.34,;12.83,-5.11,;12.83,-6.66,;14.16,-7.43,;15.49,-6.66,;16.97,-7.14,;17.88,-5.88,;16.97,-4.63,;15.49,-5.11,;19.42,-5.88,;20.19,-7.22,;21.73,-7.22,;22.5,-5.88,;21.72,-4.54,;20.18,-4.55,;17.45,-8.6,;18.95,-8.92,;19.43,-10.38,;18.4,-11.53,;16.89,-11.21,;15.64,-12.11,;14.4,-11.2,;14.88,-9.74,;16.42,-9.75,)| Show InChI InChI=1S/C31H28N8/c1-19-14-21(38-18-22-15-23(38)17-37(22)2)6-7-24(19)28-10-13-33-31-29(25-4-3-5-27-26(25)16-34-35-27)30(36-39(28)31)20-8-11-32-12-9-20/h3-14,16,22-23H,15,17-18H2,1-2H3,(H,34,35)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330918
((1R,4R)-5-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)...)Show SMILES Fc1cc(CN2C[C@H]3C[C@@H]2CO3)ccc1-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wD:9.8,7.7,(-13.19,-23.21,;-11.86,-22.44,;-11.88,-20.9,;-10.53,-20.12,;-10.54,-18.57,;-9.21,-17.8,;-7.88,-18.58,;-6.54,-17.83,;-7.7,-16.67,;-9.19,-16.26,;-7.86,-15.5,;-6.52,-16.28,;-9.19,-20.88,;-9.19,-22.42,;-10.52,-23.2,;-10.51,-24.73,;-11.85,-25.5,;-11.85,-27.05,;-10.51,-27.82,;-9.18,-27.05,;-7.7,-27.53,;-6.79,-26.27,;-7.7,-25.02,;-9.18,-25.5,;-5.25,-26.27,;-4.48,-27.61,;-2.94,-27.61,;-2.17,-26.27,;-2.95,-24.93,;-4.49,-24.94,;-7.23,-28.99,;-5.72,-29.31,;-5.24,-30.77,;-6.27,-31.92,;-7.78,-31.6,;-9.03,-32.5,;-10.27,-31.59,;-9.79,-30.13,;-8.25,-30.14,)| Show InChI InChI=1S/C30H24FN7O/c31-25-12-18(15-37-16-21-13-20(37)17-39-21)4-5-23(25)27-8-11-33-30-28(22-2-1-3-26-24(22)14-34-35-26)29(36-38(27)30)19-6-9-32-10-7-19/h1-12,14,20-21H,13,15-17H2,(H,34,35)/t20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330917
(7-(2,3-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(c(F)c1F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(1.07,-33.28,;1.07,-34.82,;-.26,-35.61,;-.25,-37.15,;.83,-36.05,;2.41,-35.59,;2.42,-37.13,;1.08,-37.91,;1.09,-39.44,;2.43,-40.21,;2.44,-41.75,;1.1,-42.52,;-.23,-41.76,;-1.56,-42.54,;-.24,-40.22,;-1.58,-39.46,;1.11,-44.06,;-.22,-44.83,;-.22,-46.37,;1.11,-47.14,;2.45,-46.37,;3.92,-46.85,;4.83,-45.6,;3.92,-44.34,;2.45,-44.82,;6.37,-45.59,;7.14,-46.93,;8.68,-46.93,;9.45,-45.59,;8.67,-44.25,;7.14,-44.26,;4.4,-48.31,;5.9,-48.63,;6.38,-50.09,;5.35,-51.25,;3.84,-50.92,;2.59,-51.82,;1.35,-50.91,;1.84,-49.45,;3.37,-49.46,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-19-13-18(38)16-39(19)25-6-5-21(27(31)28(25)32)24-9-12-34-30-26(20-3-2-4-23-22(20)14-35-36-23)29(37-40(24)30)17-7-10-33-11-8-17/h2-12,14,18-19H,13,15-16H2,1H3,(H,35,36)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330916
((1S,4S)-2-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)...)Show SMILES CN1C[C@@H]2CC[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wD:6.6,3.9,(-6.71,3.85,;-6.7,2.31,;-8.04,1.53,;-8.03,-.01,;-7.27,1.33,;-6.47,.46,;-5.36,1.55,;-5.36,.01,;-6.69,-.77,;-6.69,-2.3,;-5.35,-3.07,;-5.34,-4.61,;-6.68,-5.39,;-8.01,-4.62,;-8.02,-3.09,;-6.67,-6.92,;-8,-7.69,;-8,-9.24,;-6.67,-10.01,;-5.33,-9.24,;-3.86,-9.72,;-2.94,-8.46,;-3.86,-7.21,;-5.33,-7.69,;-1.41,-8.46,;-.64,-9.8,;.9,-9.8,;1.68,-8.46,;.9,-7.12,;-.64,-7.13,;-3.38,-11.18,;-1.88,-11.5,;-1.4,-12.96,;-2.43,-14.11,;-3.94,-13.79,;-5.19,-14.69,;-6.42,-13.78,;-5.94,-12.32,;-4.41,-12.33,)| Show InChI InChI=1S/C31H28N8/c1-37-18-24-10-9-23(37)19-38(24)22-7-5-20(6-8-22)28-13-16-33-31-29(25-3-2-4-27-26(25)17-34-35-27)30(36-39(28)31)21-11-14-32-15-12-21/h2-8,11-17,23-24H,9-10,18-19H2,1H3,(H,34,35)/t23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330915
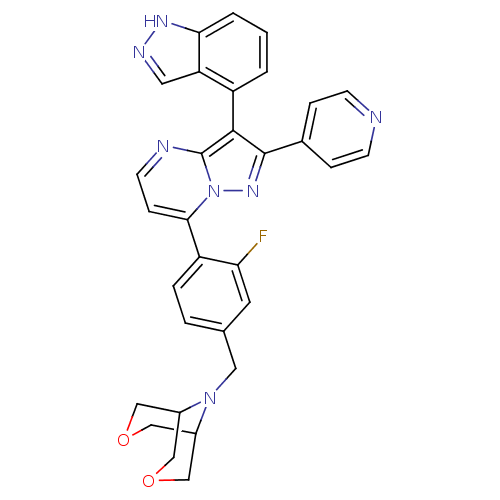
(9-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)pyrazolo...)Show SMILES Fc1cc(CN2C3COCC2COC3)ccc1-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |(13.8,-22.79,;15.13,-22.01,;15.12,-20.47,;16.4,-19.69,;16.45,-18.15,;17.8,-17.42,;18.65,-16.43,;18.72,-14.73,;20.2,-14.56,;19.38,-15.46,;19.29,-17.23,;21.11,-17.39,;21.5,-15.89,;20.4,-16.61,;17.79,-20.46,;17.8,-22,;16.46,-22.77,;16.47,-24.31,;15.14,-25.08,;15.14,-26.62,;16.47,-27.39,;17.81,-26.62,;19.28,-27.1,;20.19,-25.84,;19.28,-24.59,;17.81,-25.07,;21.73,-25.84,;22.5,-27.18,;24.04,-27.18,;24.81,-25.84,;24.03,-24.5,;22.5,-24.51,;19.76,-28.56,;21.26,-28.88,;21.74,-30.34,;20.71,-31.49,;19.2,-31.17,;17.95,-32.07,;16.71,-31.16,;17.2,-29.7,;18.73,-29.71,)| Show InChI InChI=1S/C31H26FN7O2/c32-26-12-19(14-38-21-15-40-17-22(38)18-41-16-21)4-5-24(26)28-8-11-34-31-29(23-2-1-3-27-25(23)13-35-36-27)30(37-39(28)31)20-6-9-33-10-7-20/h1-13,21-22H,14-18H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330914
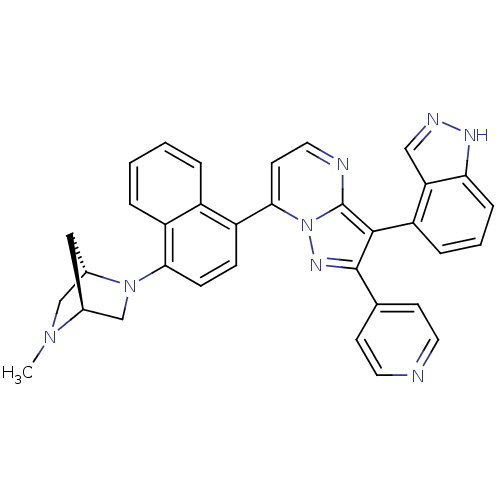
(3-(1H-indazol-4-yl)-7-(4-((1S,4S)-5-methyl-2,5-dia...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(-c2ccnc3c(c(nn23)-c2ccncc2)-c2cccc3[nH]ncc23)c2ccccc12 |r,wU:3.3,5.4,(15.66,4.33,;15.67,2.79,;14.34,2,;14.34,.46,;15.43,1.56,;17.01,2.02,;17.01,.48,;15.68,-.3,;15.69,-1.83,;17.03,-2.6,;17.03,-4.14,;15.69,-4.91,;15.7,-6.45,;14.37,-7.22,;14.38,-8.76,;15.71,-9.54,;17.04,-8.76,;18.52,-9.24,;19.43,-7.99,;18.52,-6.73,;17.04,-7.21,;20.97,-7.98,;21.74,-9.32,;23.28,-9.32,;24.05,-7.98,;23.27,-6.65,;21.73,-6.65,;18.99,-10.7,;20.5,-11.02,;20.98,-12.48,;19.94,-13.64,;18.43,-13.32,;17.19,-14.21,;15.95,-13.3,;16.43,-11.85,;17.97,-11.85,;14.37,-4.15,;13.06,-4.93,;11.72,-4.19,;11.69,-2.65,;13.01,-1.87,;14.35,-2.61,)| Show InChI InChI=1S/C34H28N8/c1-40-19-23-17-22(40)20-41(23)30-10-9-26(24-5-2-3-6-25(24)30)31-13-16-36-34-32(27-7-4-8-29-28(27)18-37-38-29)33(39-42(31)34)21-11-14-35-15-12-21/h2-16,18,22-23H,17,19-20H2,1H3,(H,37,38)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330912
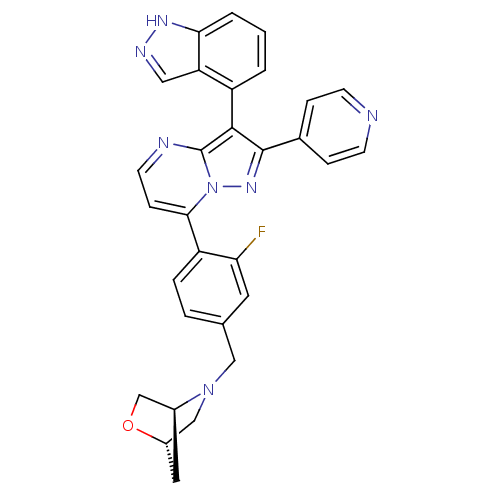
((1S,4S)-5-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)...)Show SMILES Fc1cc(CN2C[C@@H]3C[C@H]2CO3)ccc1-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:9.8,7.7,(25.62,-2.98,;26.95,-2.2,;26.94,-.66,;28.28,.12,;28.27,1.66,;29.6,2.44,;30.93,1.65,;32.27,2.41,;31.1,3.56,;29.61,3.97,;30.95,4.73,;32.29,3.95,;29.61,-.65,;29.62,-2.19,;28.28,-2.96,;28.29,-4.5,;26.96,-5.27,;26.96,-6.82,;28.3,-7.59,;29.63,-6.81,;31.1,-7.29,;32.02,-6.04,;31.1,-4.78,;29.63,-5.26,;33.55,-6.03,;34.32,-7.37,;35.86,-7.37,;36.64,-6.04,;35.86,-4.7,;34.32,-4.7,;31.58,-8.76,;33.08,-9.07,;33.56,-10.53,;32.53,-11.69,;31.02,-11.37,;29.78,-12.26,;28.54,-11.35,;29.02,-9.9,;30.55,-9.9,)| Show InChI InChI=1S/C30H24FN7O/c31-25-12-18(15-37-16-21-13-20(37)17-39-21)4-5-23(25)27-8-11-33-30-28(22-2-1-3-26-24(22)14-34-35-26)29(36-38(27)30)19-6-9-32-10-7-19/h1-12,14,20-21H,13,15-17H2,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330913
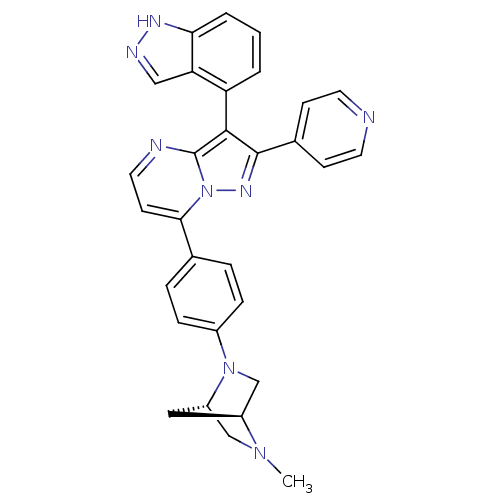
(3-(1H-indazol-4-yl)-7-(4-((1S,4S)-5-methyl-2,5-dia...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(-9.85,7.04,;-9.84,5.5,;-11.18,4.71,;-11.16,3.17,;-10.08,4.27,;-8.5,4.73,;-8.5,3.19,;-9.83,2.41,;-9.82,.88,;-8.48,.11,;-8.48,-1.43,;-9.82,-2.2,;-11.15,-1.44,;-11.16,.1,;-9.81,-3.74,;-11.14,-4.51,;-11.14,-6.05,;-9.8,-6.82,;-8.47,-6.05,;-6.99,-6.53,;-6.08,-5.28,;-6.99,-4.02,;-8.47,-4.5,;-4.54,-5.27,;-3.77,-6.61,;-2.23,-6.61,;-1.46,-5.27,;-2.24,-3.93,;-3.78,-3.94,;-6.52,-7.99,;-5.02,-8.31,;-4.53,-9.77,;-5.57,-10.93,;-7.08,-10.6,;-8.32,-11.5,;-9.56,-10.59,;-9.08,-9.13,;-7.54,-9.14,)| Show InChI InChI=1S/C30H26N8/c1-36-17-23-15-22(36)18-37(23)21-7-5-19(6-8-21)27-11-14-32-30-28(24-3-2-4-26-25(24)16-33-34-26)29(35-38(27)30)20-9-12-31-13-10-20/h2-14,16,22-23H,15,17-18H2,1H3,(H,33,34)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330911
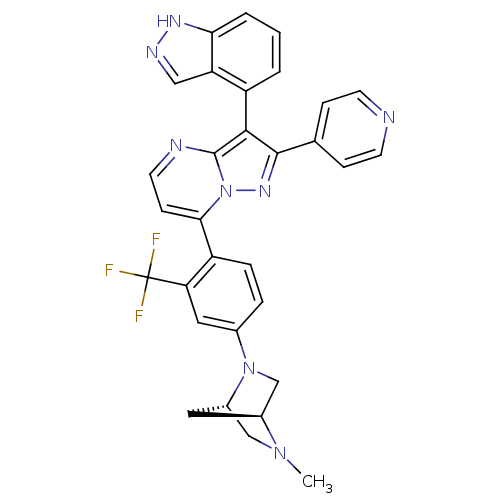
(3-(1H-indazol-4-yl)-7-(4-((1S,4S)-5-methyl-2,5-dia...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(-c2ccnc3c(c(nn23)-c2ccncc2)-c2cccc3[nH]ncc23)c(c1)C(F)(F)F |r,wU:3.3,5.4,(-.03,-12.85,;-.02,-14.39,;-1.36,-15.18,;-1.35,-16.72,;-.26,-15.62,;1.32,-15.16,;1.32,-16.7,;-.01,-17.47,;-0,-19.01,;1.33,-19.78,;1.34,-21.32,;0,-22.09,;.01,-23.62,;-1.32,-24.4,;-1.32,-25.94,;.02,-26.71,;1.35,-25.94,;2.82,-26.42,;3.74,-25.16,;2.82,-23.91,;1.35,-24.39,;5.27,-25.16,;6.04,-26.5,;7.58,-26.5,;8.35,-25.16,;7.58,-23.82,;6.04,-23.83,;3.3,-27.88,;4.8,-28.2,;5.28,-29.66,;4.25,-30.81,;2.74,-30.49,;1.49,-31.39,;.26,-30.48,;.74,-29.02,;2.27,-29.03,;-1.33,-21.33,;-1.34,-19.79,;-2.66,-22.11,;-4,-21.34,;-2.65,-23.65,;-4.16,-22.5,)| Show InChI InChI=1S/C31H25F3N8/c1-40-16-21-13-20(40)17-41(21)19-5-6-23(25(14-19)31(32,33)34)27-9-12-36-30-28(22-3-2-4-26-24(22)15-37-38-26)29(39-42(27)30)18-7-10-35-11-8-18/h2-12,14-15,20-21H,13,16-17H2,1H3,(H,37,38)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330910
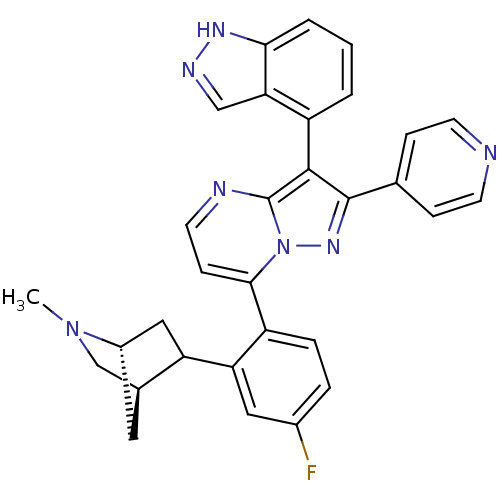
(7-(4-fluoro-2-((1R,4R)-2-methyl-2-azabicyclo[2.2.1...)Show SMILES CN1C[C@@H]2C[C@H]1CC2c1cc(F)ccc1-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(-2.11,-1.77,;-.77,-1,;.57,-1.77,;1.9,-.99,;.41,-.6,;-.78,.54,;.56,1.32,;1.89,.54,;3.22,1.31,;3.2,2.85,;4.53,3.63,;4.53,5.17,;5.87,2.86,;5.88,1.32,;4.54,.55,;4.55,-.99,;3.22,-1.76,;3.22,-3.31,;4.55,-4.08,;5.89,-3.3,;7.36,-3.78,;8.27,-2.53,;7.36,-1.27,;5.89,-1.75,;9.81,-2.52,;10.58,-3.86,;12.12,-3.86,;12.89,-2.53,;12.12,-1.19,;10.58,-1.19,;7.84,-5.25,;9.34,-5.56,;9.82,-7.02,;8.79,-8.18,;7.28,-7.86,;6.03,-8.75,;4.8,-7.84,;5.28,-6.39,;6.81,-6.39,)| Show InChI InChI=1S/C31H26FN7/c1-38-17-19-13-21(38)15-24(19)25-14-20(32)5-6-22(25)28-9-12-34-31-29(23-3-2-4-27-26(23)16-35-36-27)30(37-39(28)31)18-7-10-33-11-8-18/h2-12,14,16,19,21,24H,13,15,17H2,1H3,(H,35,36)/t19-,21-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330909
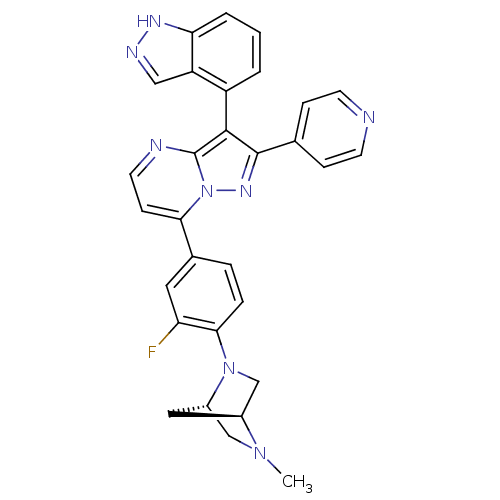
(7-(3-fluoro-4-((1S,4S)-5-methyl-2,5-diazabicyclo[2...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(22.69,-13.24,;22.7,-14.78,;21.37,-15.56,;21.38,-17.1,;22.46,-16,;24.04,-15.54,;24.05,-17.08,;22.71,-17.86,;22.72,-19.39,;24.06,-20.16,;24.06,-21.7,;22.73,-22.47,;21.39,-21.71,;21.38,-20.17,;20.04,-19.41,;22.74,-24.01,;21.41,-24.78,;21.4,-26.33,;22.74,-27.1,;24.07,-26.32,;25.55,-26.8,;26.46,-25.55,;25.55,-24.29,;24.07,-24.77,;28,-25.54,;28.77,-26.88,;30.31,-26.88,;31.08,-25.55,;30.3,-24.21,;28.76,-24.21,;26.02,-28.27,;27.53,-28.58,;28.01,-30.04,;26.98,-31.2,;25.47,-30.88,;24.22,-31.77,;22.98,-30.87,;23.46,-29.41,;25,-29.41,)| Show InChI InChI=1S/C30H25FN8/c1-37-16-21-14-20(37)17-38(21)27-6-5-19(13-24(27)31)26-9-12-33-30-28(22-3-2-4-25-23(22)15-34-35-25)29(36-39(26)30)18-7-10-32-11-8-18/h2-13,15,20-21H,14,16-17H2,1H3,(H,34,35)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330908
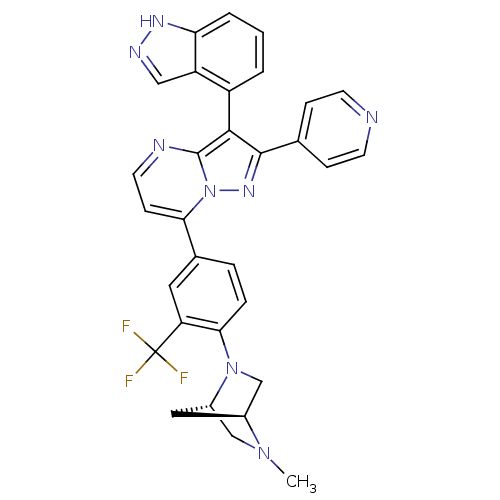
(3-(1H-indazol-4-yl)-7-(4-((1S,4S)-5-methyl-2,5-dia...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1ccc(cc1C(F)(F)F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(34.33,-13.96,;34.34,-15.5,;33,-16.29,;33.02,-17.82,;34.1,-16.72,;35.68,-16.27,;35.69,-17.81,;34.35,-18.58,;34.36,-20.12,;35.7,-20.88,;35.7,-22.42,;34.37,-23.2,;33.03,-22.44,;33.02,-20.9,;31.68,-20.14,;31.67,-18.59,;30.35,-20.92,;30.34,-19.36,;34.38,-24.73,;33.05,-25.5,;33.04,-27.05,;34.38,-27.82,;35.71,-27.05,;37.19,-27.53,;38.1,-26.27,;37.19,-25.02,;35.71,-25.5,;39.64,-26.27,;40.41,-27.61,;41.95,-27.61,;42.72,-26.27,;41.94,-24.93,;40.4,-24.94,;37.66,-28.99,;39.17,-29.31,;39.65,-30.77,;38.61,-31.92,;37.11,-31.6,;35.86,-32.5,;34.62,-31.59,;35.1,-30.13,;36.64,-30.14,)| Show InChI InChI=1S/C31H25F3N8/c1-40-16-21-14-20(40)17-41(21)27-6-5-19(13-24(27)31(32,33)34)26-9-12-36-30-28(22-3-2-4-25-23(22)15-37-38-25)29(39-42(26)30)18-7-10-35-11-8-18/h2-13,15,20-21H,14,16-17H2,1H3,(H,37,38)/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330907
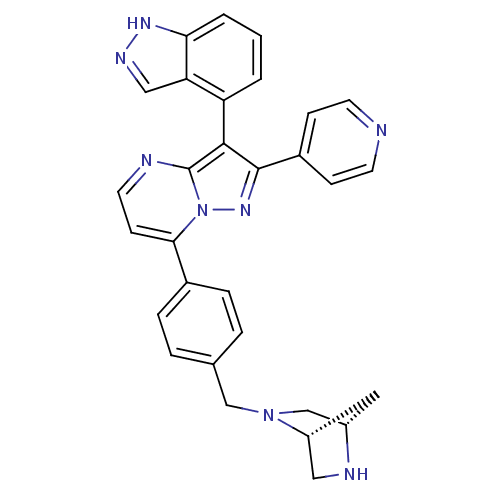
(7-(4-((1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-ylme...)Show SMILES C(N1C[C@@H]2C[C@@H]1CN2)c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,wD:5.4,(3.49,-2.92,;4.83,-2.15,;6.16,-2.93,;7.49,-2.16,;6.31,-1.02,;4.82,-.62,;6.15,.15,;7.71,-.52,;3.5,-4.46,;4.84,-5.23,;4.84,-6.77,;3.5,-7.54,;2.18,-6.79,;2.16,-5.24,;3.52,-9.08,;2.19,-9.85,;2.18,-11.4,;3.52,-12.17,;4.85,-11.39,;6.33,-11.87,;7.24,-10.61,;6.33,-9.37,;4.85,-9.84,;8.77,-10.62,;9.54,-11.95,;11.08,-11.95,;11.85,-10.62,;11.08,-9.27,;9.53,-9.28,;6.8,-13.34,;8.3,-13.65,;8.79,-15.11,;7.75,-16.26,;6.24,-15.94,;4.99,-16.84,;3.75,-15.93,;4.23,-14.46,;5.77,-14.47,)| Show InChI InChI=1S/C30H26N8/c1-2-24(25-16-34-35-26(25)3-1)28-29(21-8-11-31-12-9-21)36-38-27(10-13-32-30(28)38)20-6-4-19(5-7-20)17-37-18-22-14-23(37)15-33-22/h1-13,16,22-23,33H,14-15,17-18H2,(H,34,35)/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470911

(CHEMBL122825)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2csc(c2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C23H30N2O8S/c1-11(5-19-24-8-18(32-19)15-7-20(25(29)30)34-10-15)4-16-22(28)21(27)14(9-31-16)6-17-23(33-17)12(2)13(3)26/h5,7-8,10,12-14,16-17,21-23,26-28H,4,6,9H2,1-3H3/b11-5+/t12-,13-,14-,16-,17-,21+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.45 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470914
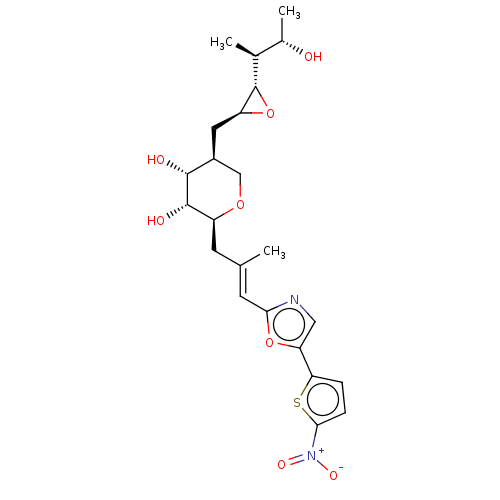
(CHEMBL120343)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2ccc(s2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C23H30N2O8S/c1-11(7-19-24-9-17(32-19)18-4-5-20(34-18)25(29)30)6-15-22(28)21(27)14(10-31-15)8-16-23(33-16)12(2)13(3)26/h4-5,7,9,12-16,21-23,26-28H,6,8,10H2,1-3H3/b11-7+/t12-,13-,14-,15-,16-,21+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.67 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus NCTC 3571 |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470913
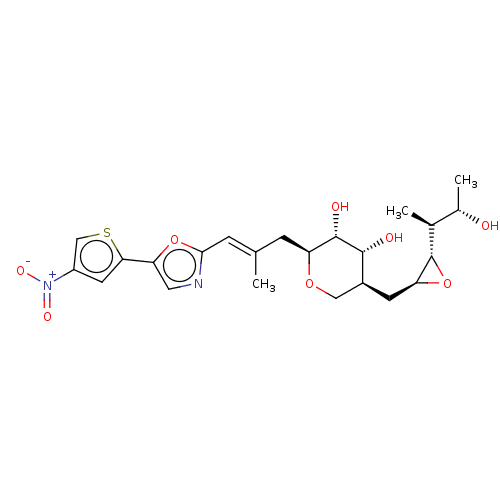
(CHEMBL120798)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2cc(cs2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C23H30N2O8S/c1-11(5-20-24-8-18(32-20)19-7-15(10-34-19)25(29)30)4-16-22(28)21(27)14(9-31-16)6-17-23(33-17)12(2)13(3)26/h5,7-8,10,12-14,16-17,21-23,26-28H,4,6,9H2,1-3H3/b11-5+/t12-,13-,14-,16-,17-,21+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.87 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330906
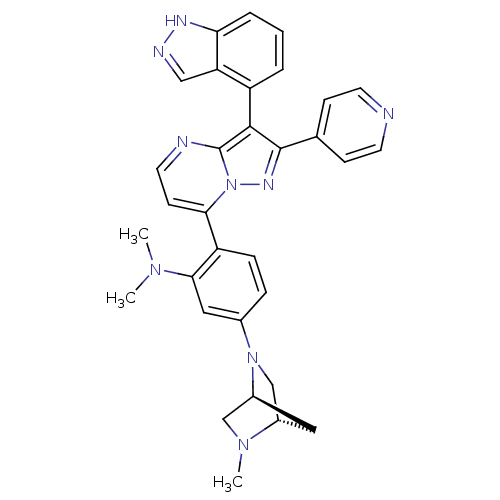
(2-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)pyrazolo[1,...)Show SMILES CN(C)c1cc(ccc1-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12)N1C[C@@H]2C[C@H]1CN2C |r,wU:37.42,35.41,(6.85,-21.06,;8.19,-21.82,;8.2,-23.36,;9.52,-21.04,;9.51,-19.5,;10.85,-18.72,;12.19,-19.49,;12.19,-21.03,;10.85,-21.8,;10.86,-23.34,;9.53,-24.11,;9.53,-25.66,;10.87,-26.43,;12.2,-25.66,;13.68,-26.13,;14.59,-24.88,;13.68,-23.62,;12.2,-24.1,;16.13,-24.88,;16.9,-26.21,;18.44,-26.21,;19.21,-24.88,;18.43,-23.54,;16.89,-23.54,;14.15,-27.6,;15.65,-27.91,;16.14,-29.37,;15.1,-30.53,;13.59,-30.21,;12.35,-31.1,;11.11,-30.2,;11.59,-28.74,;13.13,-28.75,;10.84,-17.19,;12.17,-16.41,;12.17,-14.87,;10.59,-15.33,;9.51,-16.43,;9.49,-14.89,;10.83,-14.11,;10.82,-12.57,)| Show InChI InChI=1S/C32H31N9/c1-38(2)29-16-21(40-19-22-15-23(40)18-39(22)3)7-8-25(29)28-11-14-34-32-30(24-5-4-6-27-26(24)17-35-36-27)31(37-41(28)32)20-9-12-33-13-10-20/h4-14,16-17,22-23H,15,18-19H2,1-3H3,(H,35,36)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Staphylococcus aureus) | BDBM50470912

(CHEMBL120437)Show SMILES [H][C@]1(O[C@H]1C[C@H]1CO[C@@H](C\C(C)=C\c2ncc(o2)-c2ccc(cc2)[N+]([O-])=O)[C@H](O)[C@@H]1O)[C@@H](C)[C@H](C)O Show InChI InChI=1S/C25H32N2O8/c1-13(9-22-26-11-21(34-22)16-4-6-18(7-5-16)27(31)32)8-19-24(30)23(29)17(12-33-19)10-20-25(35-20)14(2)15(3)28/h4-7,9,11,14-15,17,19-20,23-25,28-30H,8,10,12H2,1-3H3/b13-9+/t14-,15-,17-,19-,20-,23+,24-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.98 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against isoleucyl-tRNA synthetase (IRS) of Staphylococcus aureus |
J Med Chem 39: 3596-600 (1996)
Article DOI: 10.1021/jm950882q
BindingDB Entry DOI: 10.7270/Q2TX3J3G |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50330905

(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(24.13,-33.61,;24.13,-35.15,;22.8,-35.94,;22.81,-37.47,;23.89,-36.37,;25.47,-35.92,;25.48,-37.46,;24.14,-38.23,;24.15,-39.77,;22.82,-40.55,;22.83,-42.09,;21.5,-42.86,;24.16,-42.85,;25.5,-42.07,;26.83,-42.84,;25.49,-40.53,;24.17,-44.38,;22.84,-45.15,;22.84,-46.7,;24.17,-47.47,;25.51,-46.7,;26.98,-47.18,;27.89,-45.92,;26.98,-44.67,;25.51,-45.15,;29.43,-45.92,;30.2,-47.26,;31.74,-47.26,;32.51,-45.92,;31.73,-44.58,;30.2,-44.59,;27.46,-48.64,;28.96,-48.96,;29.44,-50.42,;28.41,-51.57,;26.9,-51.25,;25.65,-52.15,;24.41,-51.24,;24.9,-49.78,;26.43,-49.79,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-11-19(38)16-39(20)18-12-23(31)28(24(32)13-18)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,19-20H,11,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-raf |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330904
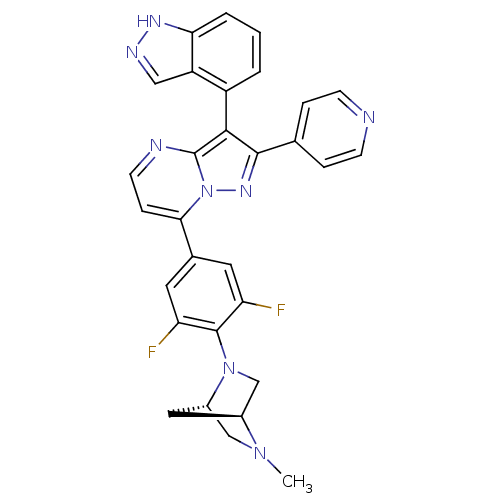
(7-(3,5-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1c(F)cc(cc1F)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(36.72,-34.69,;36.73,-36.23,;35.39,-37.02,;35.41,-38.56,;36.49,-37.45,;38.07,-37,;38.08,-38.54,;36.74,-39.31,;36.75,-40.85,;38.09,-41.62,;39.42,-40.84,;38.09,-43.16,;36.76,-43.93,;35.42,-43.17,;35.41,-41.63,;34.07,-40.87,;36.77,-45.47,;35.44,-46.24,;35.43,-47.78,;36.77,-48.55,;38.1,-47.78,;39.58,-48.26,;40.49,-47,;39.58,-45.75,;38.1,-46.23,;42.03,-47,;42.8,-48.34,;44.34,-48.34,;45.11,-47,;44.33,-45.66,;42.79,-45.67,;40.05,-49.72,;41.56,-50.04,;42.04,-51.5,;41,-52.65,;39.5,-52.33,;38.25,-53.23,;37.01,-52.32,;37.49,-50.86,;39.03,-50.87,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-13-19(38)16-39(20)29-23(31)11-18(12-24(29)32)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)28(37-40(26)30)17-5-8-33-9-6-17/h2-12,14,19-20H,13,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330903
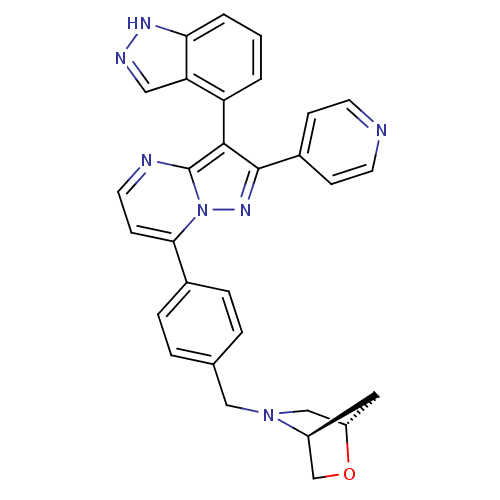
((1S,4S)-5-(4-(3-(1H-indazol-4-yl)-2-(pyridin-4-yl)...)Show SMILES C(N1C[C@@H]2C[C@H]1CO2)c1ccc(cc1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:5.4,3.3,(16.44,2.06,;17.77,2.83,;19.1,2.05,;20.44,2.8,;19.27,3.96,;17.78,4.37,;19.12,5.13,;20.46,4.35,;16.45,.52,;17.78,-.25,;17.79,-1.79,;16.45,-2.57,;15.12,-1.8,;15.11,-.27,;16.46,-4.1,;15.13,-4.87,;15.13,-6.42,;16.47,-7.19,;17.8,-6.42,;19.27,-6.9,;20.19,-5.64,;19.27,-4.39,;17.8,-4.87,;21.72,-5.64,;22.49,-6.98,;24.03,-6.98,;24.81,-5.64,;24.03,-4.3,;22.49,-4.31,;19.75,-8.36,;21.25,-8.68,;21.73,-10.14,;20.7,-11.29,;19.19,-10.97,;17.95,-11.87,;16.71,-10.96,;17.19,-9.5,;18.72,-9.51,)| Show InChI InChI=1S/C30H25N7O/c1-2-24(25-15-33-34-26(25)3-1)28-29(21-8-11-31-12-9-21)35-37-27(10-13-32-30(28)37)20-6-4-19(5-7-20)16-36-17-23-14-22(36)18-38-23/h1-13,15,22-23H,14,16-18H2,(H,33,34)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29511
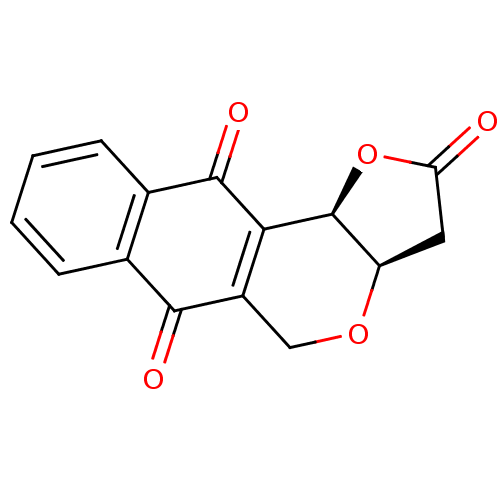
(Pyranonaphthoquinone (PNQ) lactone, 11a)Show SMILES O=C1C[C@H]2OCC3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:6| Show InChI InChI=1S/C15H10O5/c16-11-5-10-15(20-11)12-9(6-19-10)13(17)7-3-1-2-4-8(7)14(12)18/h1-4,10,15H,5-6H2/t10-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29512
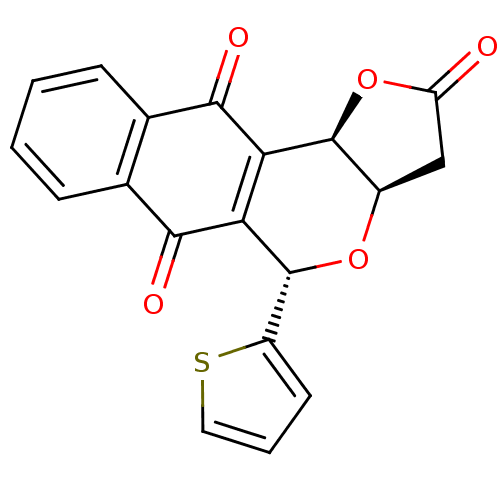
(Pyranonaphthoquinone (PNQ) lactone, 11b)Show SMILES O=C1C[C@H]2O[C@H](c3cccs3)C3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:12| Show InChI InChI=1S/C19H12O5S/c20-13-8-11-18(24-13)14-15(19(23-11)12-6-3-7-25-12)17(22)10-5-2-1-4-9(10)16(14)21/h1-7,11,18-19H,8H2/t11-,18+,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330902
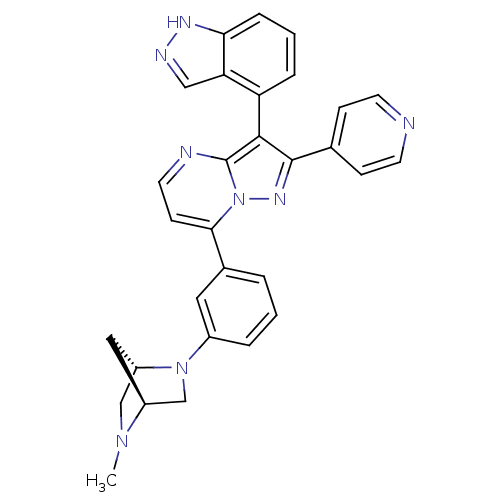
(3-(1H-indazol-4-yl)-7-(3-((1S,4S)-5-methyl-2,5-dia...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cccc(c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(10.64,4.14,;11.97,3.38,;11.98,1.83,;13.31,1.06,;12.91,2.55,;13.31,4.15,;14.64,3.37,;14.64,1.83,;15.98,1.07,;17.32,1.85,;18.66,1.09,;18.66,-.45,;17.32,-1.23,;16,-.47,;17.33,-2.76,;16,-3.53,;16,-5.08,;17.34,-5.85,;18.67,-5.08,;20.15,-5.56,;21.06,-4.3,;20.15,-3.05,;18.67,-3.53,;22.6,-4.3,;23.37,-5.64,;24.91,-5.64,;25.68,-4.3,;24.9,-2.96,;23.36,-2.97,;20.62,-7.02,;22.12,-7.34,;22.61,-8.8,;21.57,-9.95,;20.06,-9.63,;18.82,-10.53,;17.58,-9.62,;18.06,-8.16,;19.6,-8.17,)| Show InChI InChI=1S/C30H26N8/c1-36-17-23-15-22(36)18-37(23)21-5-2-4-20(14-21)27-10-13-32-30-28(24-6-3-7-26-25(24)16-33-34-26)29(35-38(27)30)19-8-11-31-12-9-19/h2-14,16,22-23H,15,17-18H2,1H3,(H,33,34)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29513
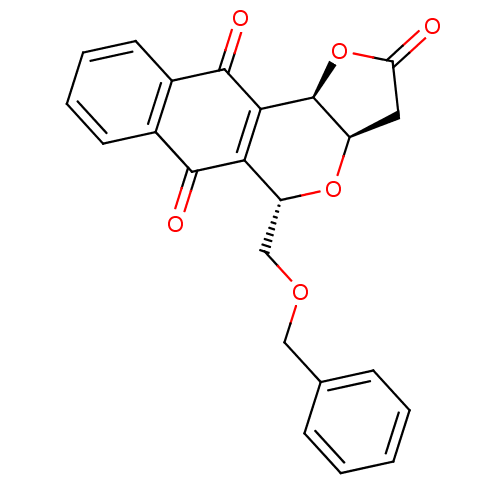
(Pyranonaphthoquinone (PNQ) lactone, 11c)Show SMILES O=C1C[C@H]2O[C@H](COCc3ccccc3)C3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:16| Show InChI InChI=1S/C23H18O6/c24-18-10-16-23(29-18)20-19(21(25)14-8-4-5-9-15(14)22(20)26)17(28-16)12-27-11-13-6-2-1-3-7-13/h1-9,16-17,23H,10-12H2/t16-,17-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29514
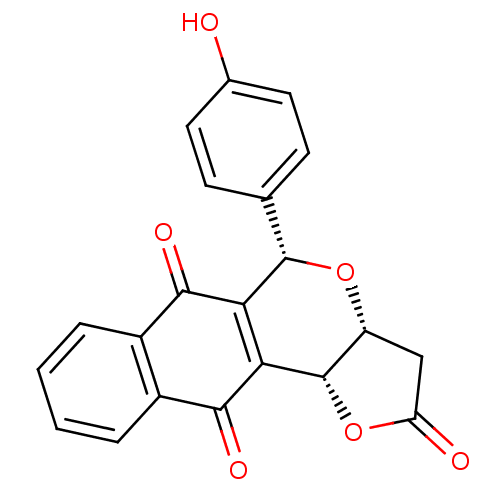
(Pyranonaphthoquinone (PNQ) lactone, 11d)Show SMILES Oc1ccc(cc1)[C@@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1ccccc1C2=O |r,c:17| Show InChI InChI=1S/C21H14O6/c22-11-7-5-10(6-8-11)20-16-17(21-14(26-20)9-15(23)27-21)19(25)13-4-2-1-3-12(13)18(16)24/h1-8,14,20-22H,9H2/t14-,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 1
(Homo sapiens (Human)) | BDBM50330905

(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(24.13,-33.61,;24.13,-35.15,;22.8,-35.94,;22.81,-37.47,;23.89,-36.37,;25.47,-35.92,;25.48,-37.46,;24.14,-38.23,;24.15,-39.77,;22.82,-40.55,;22.83,-42.09,;21.5,-42.86,;24.16,-42.85,;25.5,-42.07,;26.83,-42.84,;25.49,-40.53,;24.17,-44.38,;22.84,-45.15,;22.84,-46.7,;24.17,-47.47,;25.51,-46.7,;26.98,-47.18,;27.89,-45.92,;26.98,-44.67,;25.51,-45.15,;29.43,-45.92,;30.2,-47.26,;31.74,-47.26,;32.51,-45.92,;31.73,-44.58,;30.2,-44.59,;27.46,-48.64,;28.96,-48.96,;29.44,-50.42,;28.41,-51.57,;26.9,-51.25,;25.65,-52.15,;24.41,-51.24,;24.9,-49.78,;26.43,-49.79,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-11-19(38)16-39(20)18-12-23(31)28(24(32)13-18)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,19-20H,11,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA1 |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29515
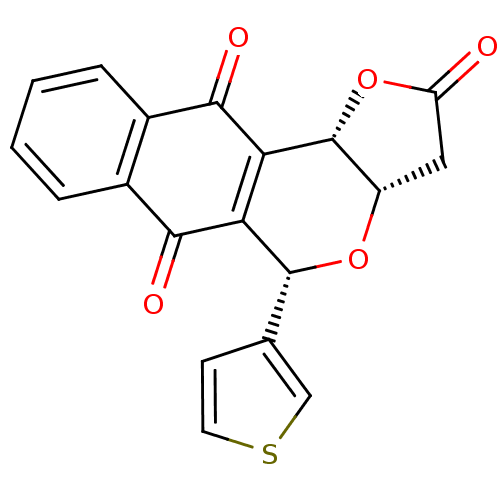
(Pyranonaphthoquinone (PNQ) lactone, 11e)Show SMILES O=C1C[C@@H]2O[C@H](c3ccsc3)C3=C([C@@H]2O1)C(=O)c1ccccc1C3=O |r,c:12| Show InChI InChI=1S/C19H12O5S/c20-13-7-12-19(24-13)15-14(18(23-12)9-5-6-25-8-9)16(21)10-3-1-2-4-11(10)17(15)22/h1-6,8,12,18-19H,7H2/t12-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29516
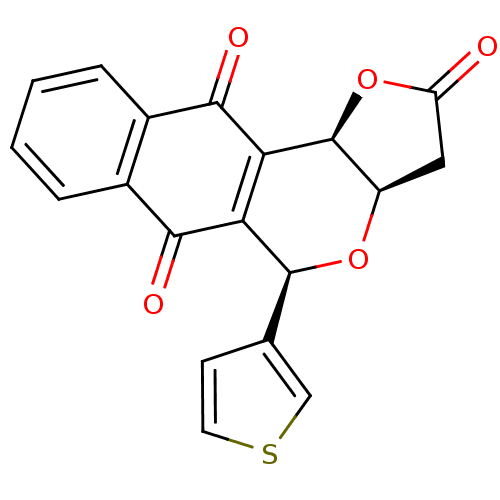
(Pyranonaphthoquinone (PNQ) lactone, 11f)Show SMILES O=C1C[C@H]2O[C@@H](c3ccsc3)C3=C([C@H]2O1)C(=O)c1ccccc1C3=O |r,c:12| Show InChI InChI=1S/C19H12O5S/c20-13-7-12-19(24-13)15-14(18(23-12)9-5-6-25-8-9)16(21)10-3-1-2-4-11(10)17(15)22/h1-6,8,12,18-19H,7H2/t12-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50330905

(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(24.13,-33.61,;24.13,-35.15,;22.8,-35.94,;22.81,-37.47,;23.89,-36.37,;25.47,-35.92,;25.48,-37.46,;24.14,-38.23,;24.15,-39.77,;22.82,-40.55,;22.83,-42.09,;21.5,-42.86,;24.16,-42.85,;25.5,-42.07,;26.83,-42.84,;25.49,-40.53,;24.17,-44.38,;22.84,-45.15,;22.84,-46.7,;24.17,-47.47,;25.51,-46.7,;26.98,-47.18,;27.89,-45.92,;26.98,-44.67,;25.51,-45.15,;29.43,-45.92,;30.2,-47.26,;31.74,-47.26,;32.51,-45.92,;31.73,-44.58,;30.2,-44.59,;27.46,-48.64,;28.96,-48.96,;29.44,-50.42,;28.41,-51.57,;26.9,-51.25,;25.65,-52.15,;24.41,-51.24,;24.9,-49.78,;26.43,-49.79,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-11-19(38)16-39(20)18-12-23(31)28(24(32)13-18)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,19-20H,11,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAPK14 |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29506
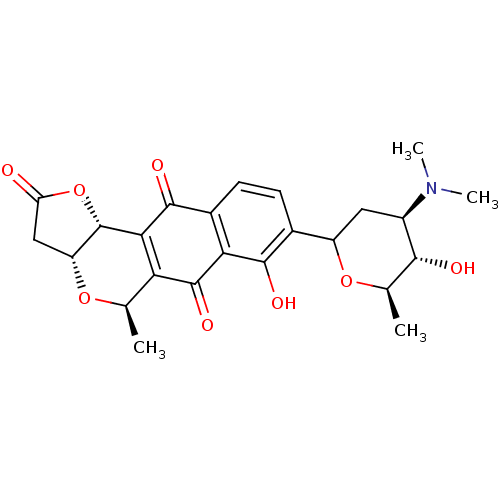
(Lactoquinomycin | Lactoquinomycin A)Show SMILES C[C@H]1OC(C[C@H]([C@@H]1O)N(C)C)c1ccc2C(=O)C3=C([C@@H](C)O[C@@H]4CC(=O)O[C@H]34)C(=O)c2c1O |r,t:18| Show InChI InChI=1S/C24H27NO8/c1-9-17-19(24-15(31-9)8-16(26)33-24)22(29)12-6-5-11(21(28)18(12)23(17)30)14-7-13(25(3)4)20(27)10(2)32-14/h5-6,9-10,13-15,20,24,27-28H,7-8H2,1-4H3/t9-,10-,13-,14?,15-,20-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29517
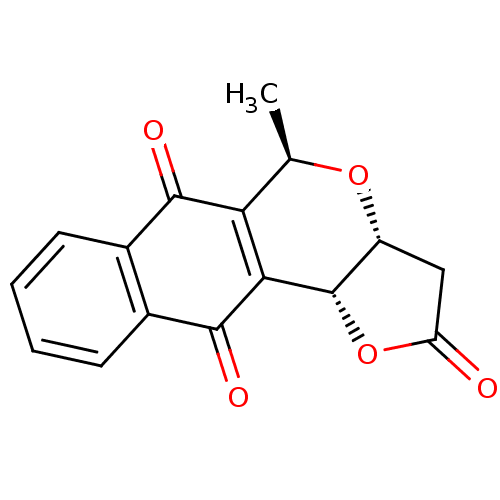
(Pyranonaphthoquinone (PNQ) lactone, 11g)Show SMILES C[C@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1ccccc1C2=O |r,c:10| Show InChI InChI=1S/C16H12O5/c1-7-12-13(16-10(20-7)6-11(17)21-16)15(19)9-5-3-2-4-8(9)14(12)18/h2-5,7,10,16H,6H2,1H3/t7-,10-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50330905

(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(24.13,-33.61,;24.13,-35.15,;22.8,-35.94,;22.81,-37.47,;23.89,-36.37,;25.47,-35.92,;25.48,-37.46,;24.14,-38.23,;24.15,-39.77,;22.82,-40.55,;22.83,-42.09,;21.5,-42.86,;24.16,-42.85,;25.5,-42.07,;26.83,-42.84,;25.49,-40.53,;24.17,-44.38,;22.84,-45.15,;22.84,-46.7,;24.17,-47.47,;25.51,-46.7,;26.98,-47.18,;27.89,-45.92,;26.98,-44.67,;25.51,-45.15,;29.43,-45.92,;30.2,-47.26,;31.74,-47.26,;32.51,-45.92,;31.73,-44.58,;30.2,-44.59,;27.46,-48.64,;28.96,-48.96,;29.44,-50.42,;28.41,-51.57,;26.9,-51.25,;25.65,-52.15,;24.41,-51.24,;24.9,-49.78,;26.43,-49.79,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-11-19(38)16-39(20)18-12-23(31)28(24(32)13-18)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,19-20H,11,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RET |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM29518
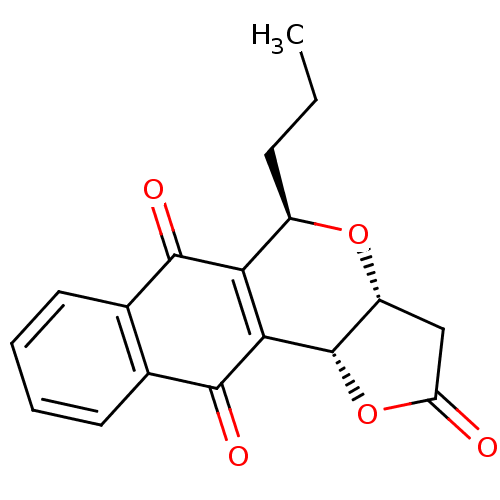
(Pyranonaphthoquinone (PNQ) lactone, 11h)Show SMILES CCC[C@H]1O[C@@H]2CC(=O)O[C@@H]2C2=C1C(=O)c1ccccc1C2=O |r,c:12| Show InChI InChI=1S/C18H16O5/c1-2-5-11-14-15(18-12(22-11)8-13(19)23-18)17(21)10-7-4-3-6-9(10)16(14)20/h3-4,6-7,11-12,18H,2,5,8H2,1H3/t11-,12-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
AKT1 was assayed using constitutively active Myr-AKT1 in low-binding 96-well plates. The kinase reactions were initiated by the addition of assay mix... |
J Med Chem 52: 2181-4 (2009)
Article DOI: 10.1021/jm900075g
BindingDB Entry DOI: 10.7270/Q2V69GW9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data