| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50333015 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_689738 (CHEMBL1635466) |
|---|
| IC50 | 96000±n/a nM |
|---|
| Citation |  Leslie, CP; Biagetti, M; Bison, S; Bromidge, SM; Fabio, RD; Donati, D; Falchi, A; Garnier, MJ; Jaxa-Chamiec, A; Manchee, G; Merlo, G; Pizzi, DA; Stasi, LP; Tibasco, J; Vong, A; Ward, SE Discovery of 1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]ethyl}phenyl)-2-imidazolidinone (GSK163090), a Potent, Selective, and Orally Active 5-HT1A/B/D Receptor Antagonist J Med Chem53:8228-8240 (2010) [PubMed] Article Leslie, CP; Biagetti, M; Bison, S; Bromidge, SM; Fabio, RD; Donati, D; Falchi, A; Garnier, MJ; Jaxa-Chamiec, A; Manchee, G; Merlo, G; Pizzi, DA; Stasi, LP; Tibasco, J; Vong, A; Ward, SE Discovery of 1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]ethyl}phenyl)-2-imidazolidinone (GSK163090), a Potent, Selective, and Orally Active 5-HT1A/B/D Receptor Antagonist J Med Chem53:8228-8240 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50333015 |
|---|
| n/a |
|---|
| Name | BDBM50333015 |
|---|
| Synonyms: | 1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]ethyl}phenyl)-2-imidazolidinone | CHEMBL1631540 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H29N5O |
|---|
| Mol. Mass. | 415.5307 |
|---|
| SMILES | Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 |
|---|
| Structure | 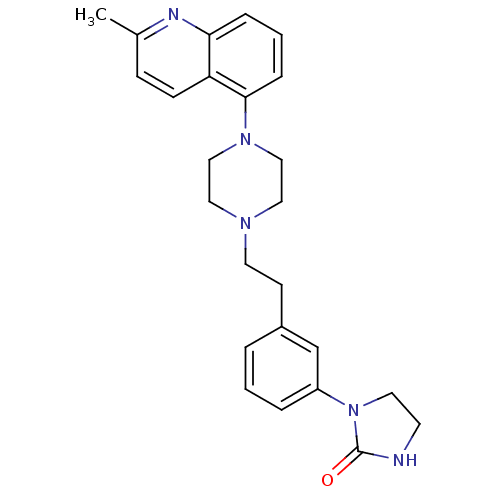
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Leslie, CP; Biagetti, M; Bison, S; Bromidge, SM; Fabio, RD; Donati, D; Falchi, A; Garnier, MJ; Jaxa-Chamiec, A; Manchee, G; Merlo, G; Pizzi, DA; Stasi, LP; Tibasco, J; Vong, A; Ward, SE Discovery of 1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]ethyl}phenyl)-2-imidazolidinone (GSK163090), a Potent, Selective, and Orally Active 5-HT1A/B/D Receptor Antagonist J Med Chem53:8228-8240 (2010) [PubMed] Article
Leslie, CP; Biagetti, M; Bison, S; Bromidge, SM; Fabio, RD; Donati, D; Falchi, A; Garnier, MJ; Jaxa-Chamiec, A; Manchee, G; Merlo, G; Pizzi, DA; Stasi, LP; Tibasco, J; Vong, A; Ward, SE Discovery of 1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]ethyl}phenyl)-2-imidazolidinone (GSK163090), a Potent, Selective, and Orally Active 5-HT1A/B/D Receptor Antagonist J Med Chem53:8228-8240 (2010) [PubMed] Article