 Found 38 hits with Last Name = 'manchee' and Initial = 'g'
Found 38 hits with Last Name = 'manchee' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM12569
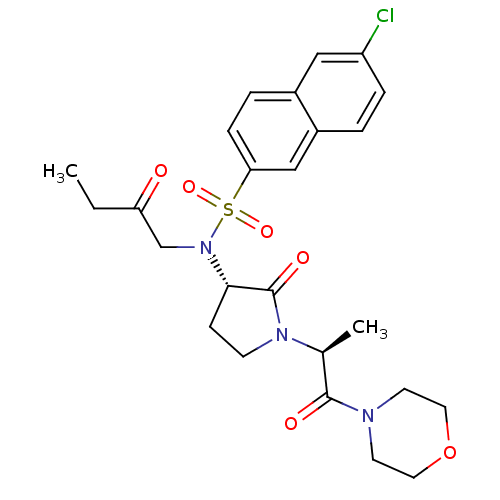
(GTC000006A | N-(6-chloronaphthalen-2-yl)-N'-[(3S)-...)Show SMILES CCC(=O)CN([C@H]1CCN([C@@H](C)C(=O)N2CCOCC2)C1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 |r| Show InChI InChI=1S/C25H30ClN3O6S/c1-3-21(30)16-29(36(33,34)22-7-5-18-14-20(26)6-4-19(18)15-22)23-8-9-28(25(23)32)17(2)24(31)27-10-12-35-13-11-27/h4-7,14-15,17,23H,3,8-13,16H2,1-2H3/t17-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12557
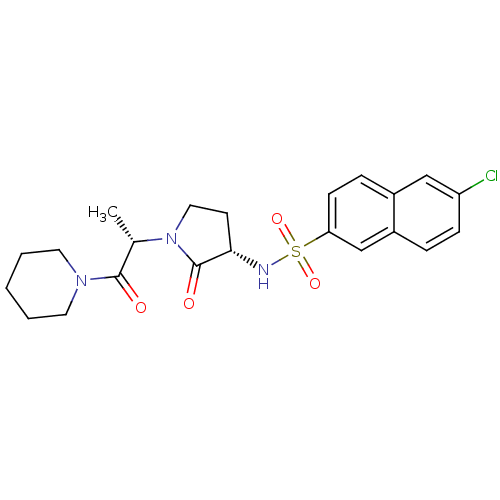
(6-chloro-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(piperidin-...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C22H26ClN3O4S/c1-15(21(27)25-10-3-2-4-11-25)26-12-9-20(22(26)28)24-31(29,30)19-8-6-16-13-18(23)7-5-17(16)14-19/h5-8,13-15,20,24H,2-4,9-12H2,1H3/t15-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12567
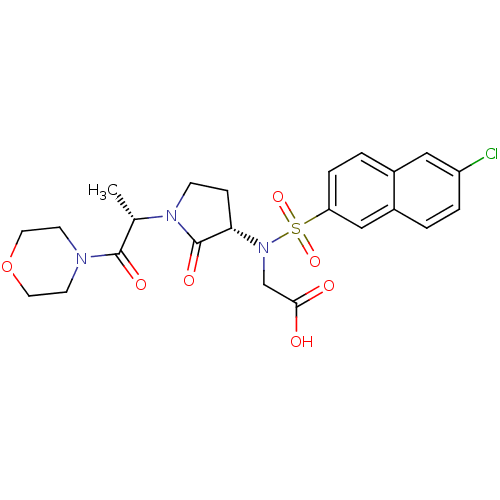
(2-[(6-chloronaphthalene-2-)[(3S)-1-[(2S)-1-(morpho...)Show SMILES C[C@H](N1CC[C@H](N(CC(O)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C23H26ClN3O7S/c1-15(22(30)25-8-10-34-11-9-25)26-7-6-20(23(26)31)27(14-21(28)29)35(32,33)19-5-3-16-12-18(24)4-2-17(16)13-19/h2-5,12-13,15,20H,6-11,14H2,1H3,(H,28,29)/t15-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12558
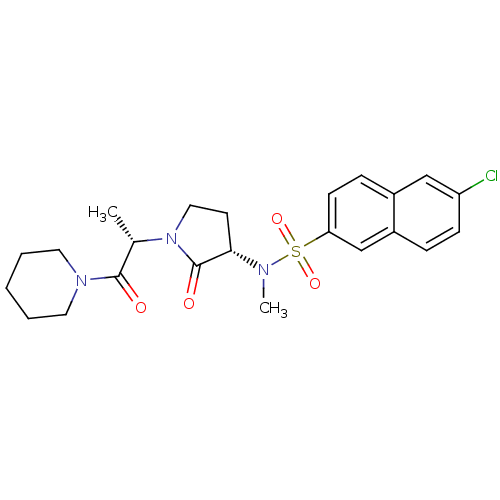
(6-chloro-N-methyl-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(p...)Show SMILES C[C@H](N1CC[C@H](N(C)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C23H28ClN3O4S/c1-16(22(28)26-11-4-3-5-12-26)27-13-10-21(23(27)29)25(2)32(30,31)20-9-7-17-14-19(24)8-6-18(17)15-20/h6-9,14-16,21H,3-5,10-13H2,1-2H3/t16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12561
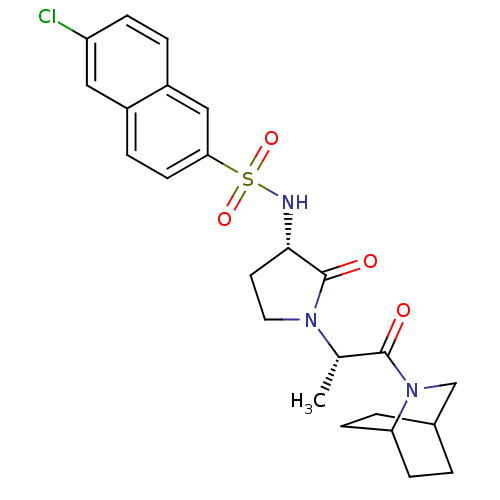
(N-[(3S)-1-[(2S)-1-{2-azabicyclo[2.2.2]octan-2-yl}-...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CC2CCC1CC2 |r,wD:1.0,5.5,(24.17,-11.35,;25.5,-12.12,;26.84,-11.35,;26.95,-9.81,;28.44,-9.44,;29.26,-10.75,;30.79,-10.86,;31.46,-12.25,;30.13,-13.02,;31.86,-13.74,;33,-12.25,;33.71,-10.88,;35.25,-10.81,;36.08,-12.11,;37.62,-12.04,;38.45,-13.34,;39.99,-13.27,;37.74,-14.71,;36.2,-14.78,;35.37,-13.48,;33.83,-13.55,;28.26,-11.93,;28.63,-13.42,;25.5,-13.66,;26.84,-14.43,;24.17,-14.43,;22.84,-13.66,;21.5,-14.43,;21.5,-15.97,;22.84,-16.74,;24.17,-15.97,;22.43,-15.88,;22.37,-15.08,)| Show InChI InChI=1S/C24H28ClN3O4S/c1-15(23(29)28-14-16-2-7-20(28)8-3-16)27-11-10-22(24(27)30)26-33(31,32)21-9-5-17-12-19(25)6-4-18(17)13-21/h4-6,9,12-13,15-16,20,22,26H,2-3,7-8,10-11,14H2,1H3/t15-,16?,20?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12568

(2-[(6-chloronaphthalene-2-)[(3S)-1-[(2S)-1-(morpho...)Show SMILES C[C@H](N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C23H27ClN4O6S/c1-15(22(30)26-8-10-34-11-9-26)27-7-6-20(23(27)31)28(14-21(25)29)35(32,33)19-5-3-16-12-18(24)4-2-17(16)13-19/h2-5,12-13,15,20H,6-11,14H2,1H3,(H2,25,29)/t15-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12538
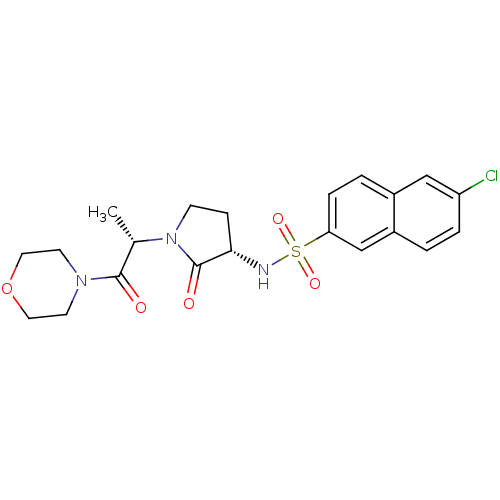
(6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C21H24ClN3O5S/c1-14(20(26)24-8-10-30-11-9-24)25-7-6-19(21(25)27)23-31(28,29)18-5-3-15-12-17(22)4-2-16(15)13-18/h2-5,12-14,19,23H,6-11H2,1H3/t14-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12566
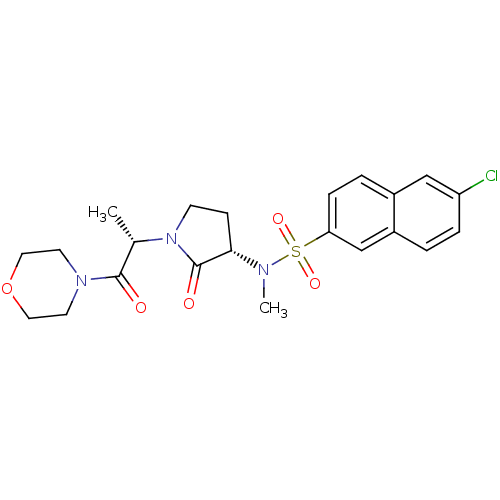
(6-chloro-N-methyl-N-[(3S)-1-[(2S)-1-(morpholin-4-y...)Show SMILES C[C@H](N1CC[C@H](N(C)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C22H26ClN3O5S/c1-15(21(27)25-9-11-31-12-10-25)26-8-7-20(22(26)28)24(2)32(29,30)19-6-4-16-13-18(23)5-3-17(16)14-19/h3-6,13-15,20H,7-12H2,1-2H3/t15-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12562
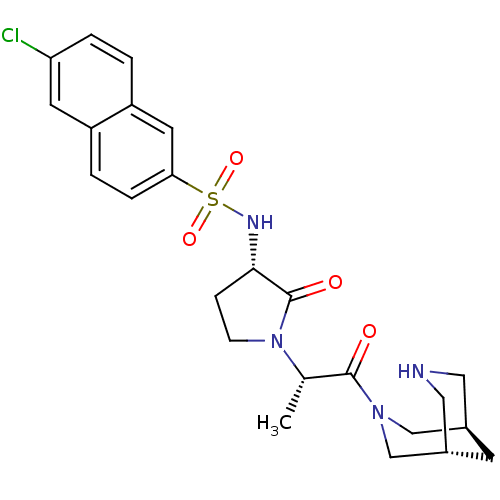
(6-chloro-N-[(3S)-1-[(2S)-1-[(1R,5S)-3,7-diazabicyc...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1C[C@H]2CNC[C@H](C2)C1 |r| Show InChI InChI=1S/C24H29ClN4O4S/c1-15(23(30)28-13-16-8-17(14-28)12-26-11-16)29-7-6-22(24(29)31)27-34(32,33)21-5-3-18-9-20(25)4-2-19(18)10-21/h2-5,9-10,15-17,22,26-27H,6-8,11-14H2,1H3/t15-,16-,17+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12574
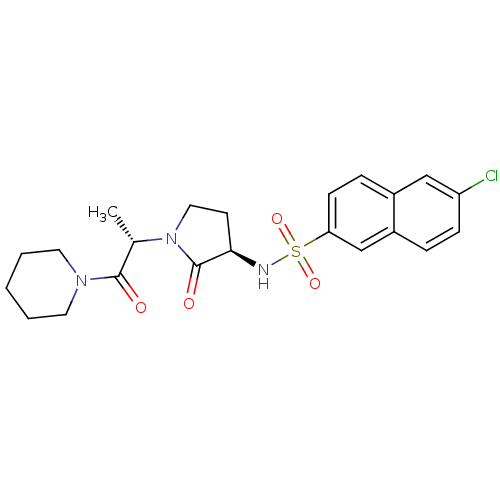
(6-chloro-N-[(3R)-2-oxo-1-[(2S)-1-oxo-1-(piperidin-...)Show SMILES C[C@H](N1CC[C@@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C22H26ClN3O4S/c1-15(21(27)25-10-3-2-4-11-25)26-12-9-20(22(26)28)24-31(29,30)19-8-6-16-13-18(23)7-5-17(16)14-19/h5-8,13-15,20,24H,2-4,9-12H2,1H3/t15-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | -44.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12573

(6-chloro-N-[(3R)-2-oxo-1-[(2R)-1-oxo-1-(piperidin-...)Show SMILES C[C@@H](N1CC[C@@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C22H26ClN3O4S/c1-15(21(27)25-10-3-2-4-11-25)26-12-9-20(22(26)28)24-31(29,30)19-8-6-16-13-18(23)7-5-17(16)14-19/h5-8,13-15,20,24H,2-4,9-12H2,1H3/t15-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12560
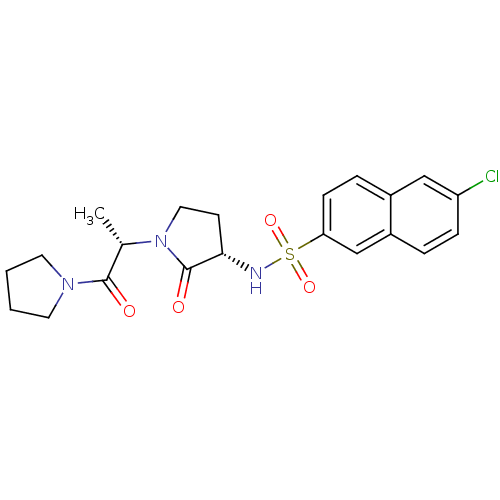
(6-chloro-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(pyrrolidin...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C21H24ClN3O4S/c1-14(20(26)24-9-2-3-10-24)25-11-8-19(21(25)27)23-30(28,29)18-7-5-15-12-17(22)6-4-16(15)13-18/h4-7,12-14,19,23H,2-3,8-11H2,1H3/t14-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50417543
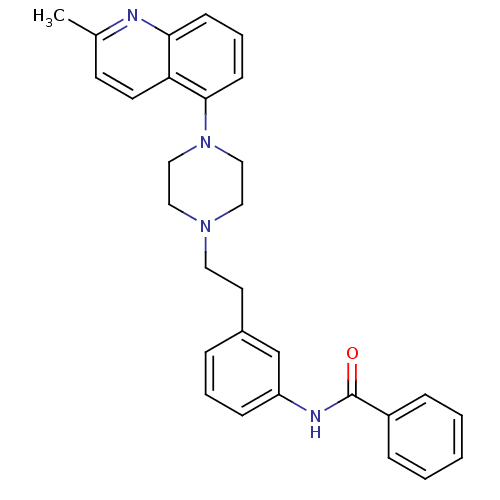
(CHEMBL1632210)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(NC(=O)c3ccccc3)c2)CC1 Show InChI InChI=1S/C29H30N4O/c1-22-13-14-26-27(30-22)11-6-12-28(26)33-19-17-32(18-20-33)16-15-23-7-5-10-25(21-23)31-29(34)24-8-3-2-4-9-24/h2-14,21H,15-20H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12571
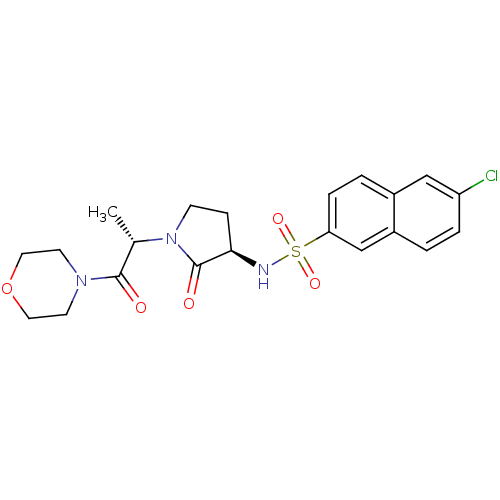
(6-chloro-N-[(3R)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...)Show SMILES C[C@H](N1CC[C@@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C21H24ClN3O5S/c1-14(20(26)24-8-10-30-11-9-24)25-7-6-19(21(25)27)23-31(28,29)18-5-3-15-12-17(22)4-2-16(15)13-18/h2-5,12-14,19,23H,6-11H2,1H3/t14-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12554
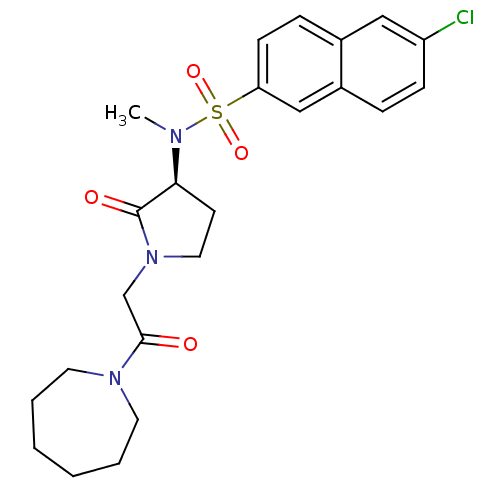
(N-[(3S)-1-[2-(azepan-1-yl)-2-oxoethyl]-2-oxopyrrol...)Show SMILES CN([C@H]1CCN(CC(=O)N2CCCCCC2)C1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 |r| Show InChI InChI=1S/C23H28ClN3O4S/c1-25(32(30,31)20-9-7-17-14-19(24)8-6-18(17)15-20)21-10-13-27(23(21)29)16-22(28)26-11-4-2-3-5-12-26/h6-9,14-15,21H,2-5,10-13,16H2,1H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12556
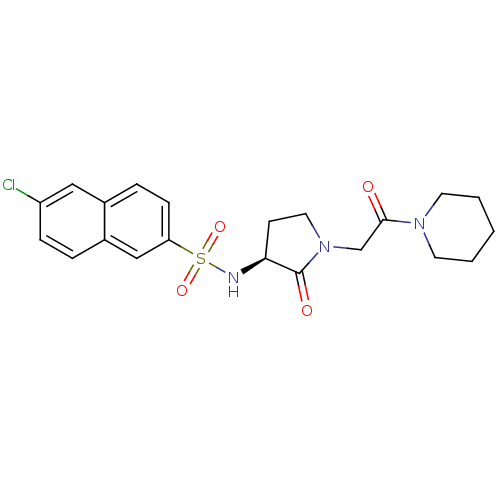
(6-chloro-N-[(3S)-2-oxo-1-[2-oxo-2-(piperidin-1-yl)...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(CC(=O)N2CCCCC2)C1=O |r| Show InChI InChI=1S/C21H24ClN3O4S/c22-17-6-4-16-13-18(7-5-15(16)12-17)30(28,29)23-19-8-11-25(21(19)27)14-20(26)24-9-2-1-3-10-24/h4-7,12-13,19,23H,1-3,8-11,14H2/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12555
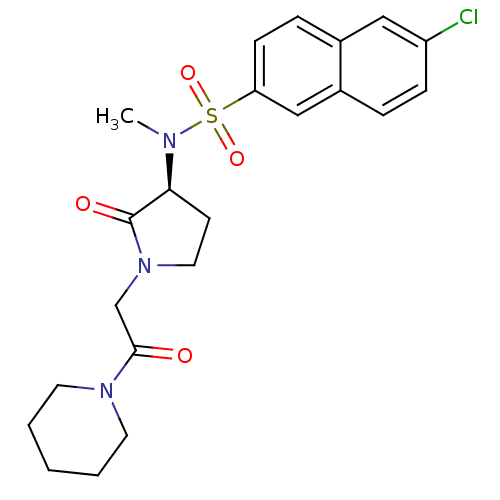
(6-chloro-N-methyl-N-[(3S)-2-oxo-1-[2-oxo-2-(piperi...)Show SMILES CN([C@H]1CCN(CC(=O)N2CCCCC2)C1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 |r| Show InChI InChI=1S/C22H26ClN3O4S/c1-24(31(29,30)19-8-6-16-13-18(23)7-5-17(16)14-19)20-9-12-26(22(20)28)15-21(27)25-10-3-2-4-11-25/h5-8,13-14,20H,2-4,9-12,15H2,1H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12575
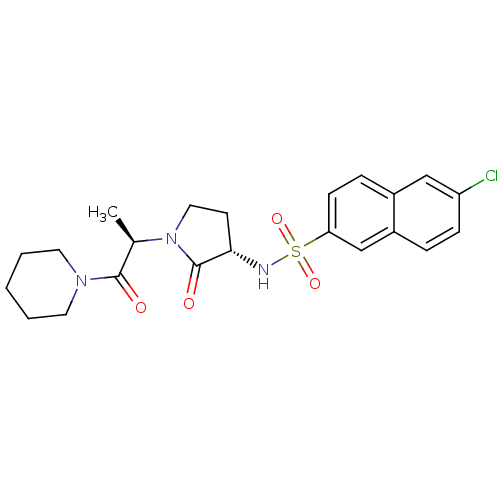
(6-chloro-N-[(3S)-2-oxo-1-[(2R)-1-oxo-1-(piperidin-...)Show SMILES C[C@@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C22H26ClN3O4S/c1-15(21(27)25-10-3-2-4-11-25)26-12-9-20(22(26)28)24-31(29,30)19-8-6-16-13-18(23)7-5-17(16)14-19/h5-8,13-15,20,24H,2-4,9-12H2,1H3/t15-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12570
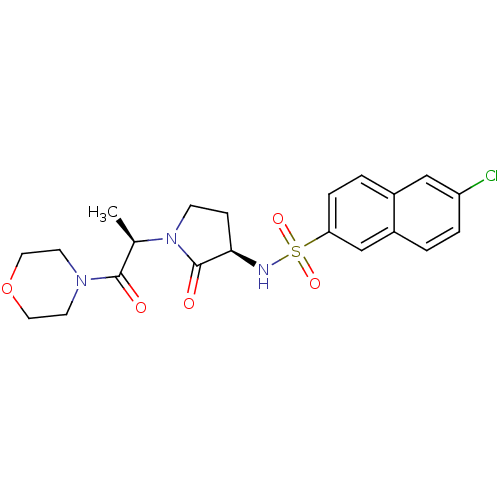
(6-chloro-N-[(3R)-1-[(2R)-1-(morpholin-4-yl)-1-oxop...)Show SMILES C[C@@H](N1CC[C@@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C21H24ClN3O5S/c1-14(20(26)24-8-10-30-11-9-24)25-7-6-19(21(25)27)23-31(28,29)18-5-3-15-12-17(22)4-2-16(15)13-18/h2-5,12-14,19,23H,6-11H2,1H3/t14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12572
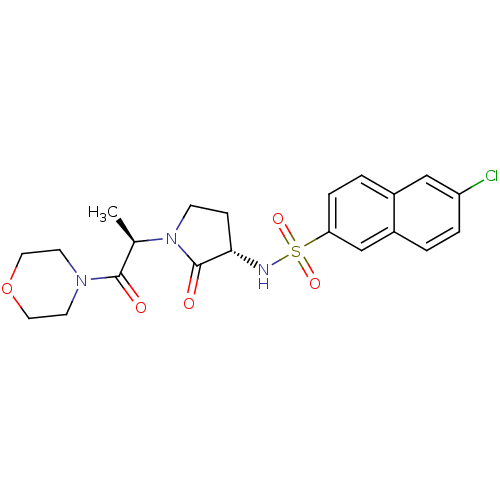
(6-chloro-N-[(3S)-1-[(2R)-1-(morpholin-4-yl)-1-oxop...)Show SMILES C[C@@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C21H24ClN3O5S/c1-14(20(26)24-8-10-30-11-9-24)25-7-6-19(21(25)27)23-31(28,29)18-5-3-15-12-17(22)4-2-16(15)13-18/h2-5,12-14,19,23H,6-11H2,1H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50417549

(CHEMBL1632223)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(NC(=O)Nc3ccccc3)c2)CC1 Show InChI InChI=1S/C29H31N5O/c1-22-13-14-26-27(30-22)11-6-12-28(26)34-19-17-33(18-20-34)16-15-23-7-5-10-25(21-23)32-29(35)31-24-8-3-2-4-9-24/h2-14,21H,15-20H2,1H3,(H2,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50417544

(CHEMBL1632218)Show SMILES Cc1nc(cs1)C(=O)Nc1cccc(CCN2CCN(CC2)c2cccc3nc(C)ccc23)c1 Show InChI InChI=1S/C27H29N5OS/c1-19-9-10-23-24(28-19)7-4-8-26(23)32-15-13-31(14-16-32)12-11-21-5-3-6-22(17-21)30-27(33)25-18-34-20(2)29-25/h3-10,17-18H,11-16H2,1-2H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12563
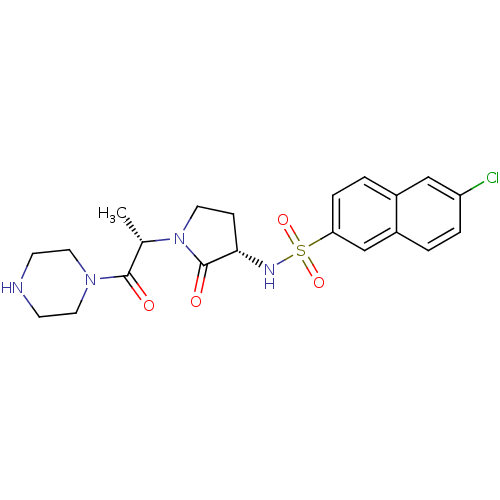
(6-chloro-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(piperazin-...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCNCC1 |r| Show InChI InChI=1S/C21H25ClN4O4S/c1-14(20(27)25-10-7-23-8-11-25)26-9-6-19(21(26)28)24-31(29,30)18-5-3-15-12-17(22)4-2-16(15)13-18/h2-5,12-14,19,23-24H,6-11H2,1H3/t14-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50417545

(CHEMBL1631535)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(NS(C)(=O)=O)c2)CC1 Show InChI InChI=1S/C23H28N4O2S/c1-18-9-10-21-22(24-18)7-4-8-23(21)27-15-13-26(14-16-27)12-11-19-5-3-6-20(17-19)25-30(2,28)29/h3-10,17,25H,11-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50417546
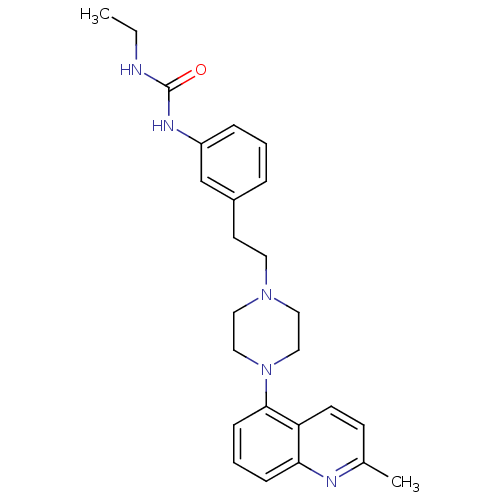
(CHEMBL1632220)Show SMILES CCNC(=O)Nc1cccc(CCN2CCN(CC2)c2cccc3nc(C)ccc23)c1 Show InChI InChI=1S/C25H31N5O/c1-3-26-25(31)28-21-7-4-6-20(18-21)12-13-29-14-16-30(17-15-29)24-9-5-8-23-22(24)11-10-19(2)27-23/h4-11,18H,3,12-17H2,1-2H3,(H2,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50417548
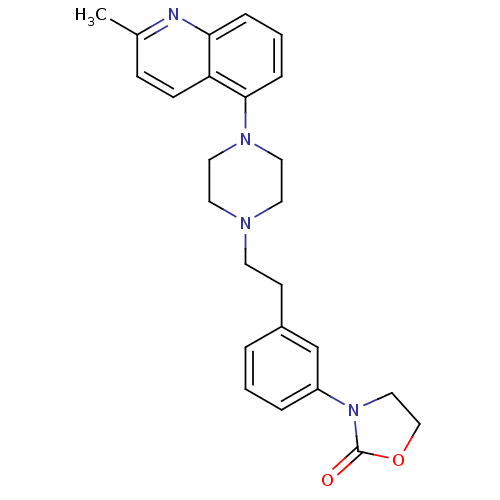
(CHEMBL1631542)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCOC2=O)CC1 Show InChI InChI=1S/C25H28N4O2/c1-19-8-9-22-23(26-19)6-3-7-24(22)28-14-12-27(13-15-28)11-10-20-4-2-5-21(18-20)29-16-17-31-25(29)30/h2-9,18H,10-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12564
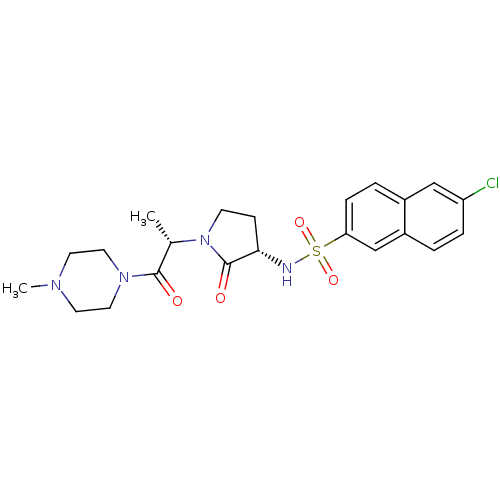
(6-chloro-N-[(3S)-1-[(2S)-1-(4-methylpiperazin-1-yl...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCN(C)CC1 |r| Show InChI InChI=1S/C22H27ClN4O4S/c1-15(21(28)26-11-9-25(2)10-12-26)27-8-7-20(22(27)29)24-32(30,31)19-6-4-16-13-18(23)5-3-17(16)14-19/h3-6,13-15,20,24H,7-12H2,1-2H3/t15-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.06E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50417547
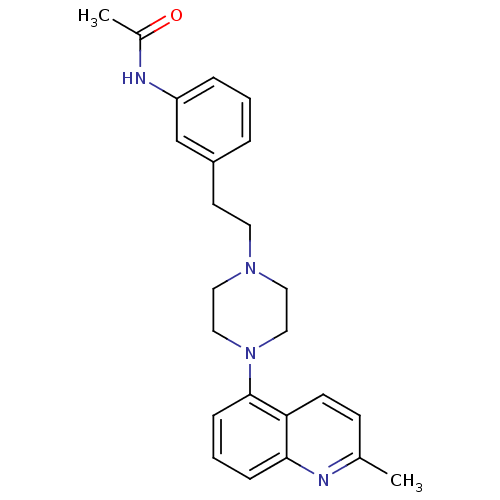
(CHEMBL1632206)Show SMILES CC(=O)Nc1cccc(CCN2CCN(CC2)c2cccc3nc(C)ccc23)c1 Show InChI InChI=1S/C24H28N4O/c1-18-9-10-22-23(25-18)7-4-8-24(22)28-15-13-27(14-16-28)12-11-20-5-3-6-21(17-20)26-19(2)29/h3-10,17H,11-16H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12559
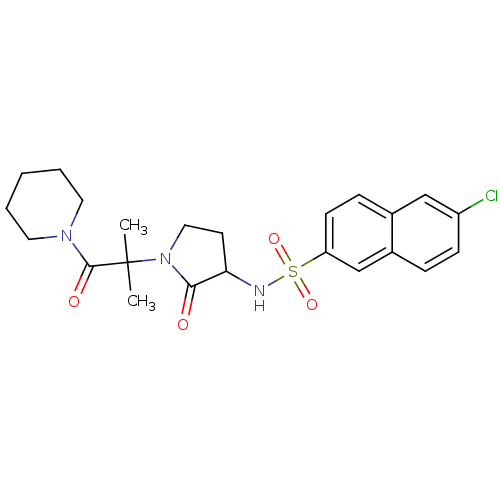
(6-chloro-N-{1-[2-methyl-1-oxo-1-(piperidin-1-yl)pr...)Show SMILES CC(C)(N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCCC1 Show InChI InChI=1S/C23H28ClN3O4S/c1-23(2,22(29)26-11-4-3-5-12-26)27-13-10-20(21(27)28)25-32(30,31)19-9-7-16-14-18(24)8-6-17(16)15-19/h6-9,14-15,20,25H,3-5,10-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50333015
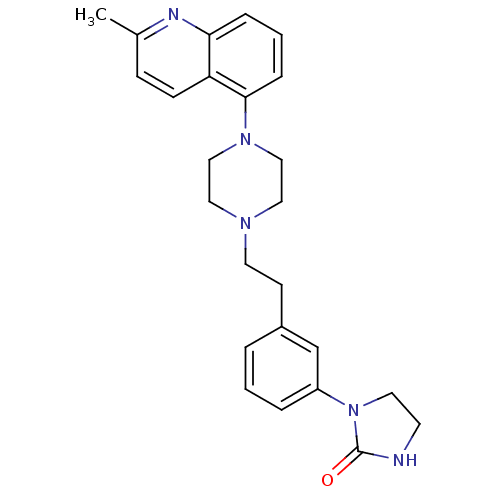
(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by SPA |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using atorvastatin as probe |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as probe |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using nifedipine as probe |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP219 |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50333015

(1-(3-{2-[4-(2-Methyl-5-quinolinyl)-1-piperazinyl]e...)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc(c2)N2CCNC2=O)CC1 Show InChI InChI=1S/C25H29N5O/c1-19-8-9-22-23(27-19)6-3-7-24(22)29-16-14-28(15-17-29)12-10-20-4-2-5-21(18-20)30-13-11-26-25(30)31/h2-9,18H,10-17H2,1H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 53: 8228-8240 (2010)
Article DOI: 10.1021/jm100714c
BindingDB Entry DOI: 10.7270/Q2SF2WD4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data