| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-C chemokine receptor type 5 |
|---|
| Ligand | BDBM50339959 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_739992 (CHEMBL1763052) |
|---|
| IC50 | 290±n/a nM |
|---|
| Citation |  Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; Langille, J; Di Fluri, M; Bogucki, D; Yang, W; Li, T; Wang, L; Nan, S; Baird, I; Metz, M; Darkes, M; Labrecque, J; Lau, G; Fricker, S; Huskens, D; Schols, D Design and synthesis of pyridin-2-yloxymethylpiperidin-1-ylbutyl amide CCR5 antagonists that are potent inhibitors of M-tropic (R5) HIV-1 replication. Bioorg Med Chem Lett21:2450-5 (2011) [PubMed] Article Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; Langille, J; Di Fluri, M; Bogucki, D; Yang, W; Li, T; Wang, L; Nan, S; Baird, I; Metz, M; Darkes, M; Labrecque, J; Lau, G; Fricker, S; Huskens, D; Schols, D Design and synthesis of pyridin-2-yloxymethylpiperidin-1-ylbutyl amide CCR5 antagonists that are potent inhibitors of M-tropic (R5) HIV-1 replication. Bioorg Med Chem Lett21:2450-5 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-C chemokine receptor type 5 |
|---|
| Name: | C-C chemokine receptor type 5 |
|---|
| Synonyms: | C-C CKR-5 | C-C chemokine receptor type 5 | CC-CKR-5 | CCR-5 | CCR5 | CCR5/mu opioid receptor complex | CCR5_HUMAN | CD_antigen=CD195 | CHEMR13 | CMKBR5 | HIV-1 fusion coreceptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 40540.21 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P51681 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MDYQVSSPIYDINYYTSEPCQKINVKQIAARLLPPLYSLVFIFGFVGNMLVILILINCKR
LKSMTDIYLLNLAISDLFFLLTVPFWAHYAAAQWDFGNTMCQLLTGLYFIGFFSGIFFII
LLTIDRYLAVVHAVFALKARTVTFGVVTSVITWVVAVFASLPGIIFTRSQKEGLHYTCSS
HFPYSQYQFWKNFQTLKIVILGLVLPLLVMVICYSGILKTLLRCRNEKKRHRAVRLIFTI
MIVYFLFWAPYNIVLLLNTFQEFFGLNNCSSSNRLDQAMQVTETLGMTHCCINPIIYAFV
GEKFRNYLLVFFQKHIAKRFCKCCSIFQQEAPERASSVYTRSTGEQEISVGL
|
|
|
|---|
| BDBM50339959 |
|---|
| n/a |
|---|
| Name | BDBM50339959 |
|---|
| Synonyms: | (E)-3-(3-(4-((4-bromophenyl)(ethoxyimino)methyl)piperidin-1-yl)butylcarbamoyl)-2,4-dimethylpyridine 1-oxide | CHEMBL1762304 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H35BrN4O3 |
|---|
| Mol. Mass. | 531.485 |
|---|
| SMILES | CCO[N-][C+](C1CCN(CC1)C(C)CCNC(=O)c1c(C)cc[n+]([O-])c1C)c1ccc(Br)cc1 |
|---|
| Structure | 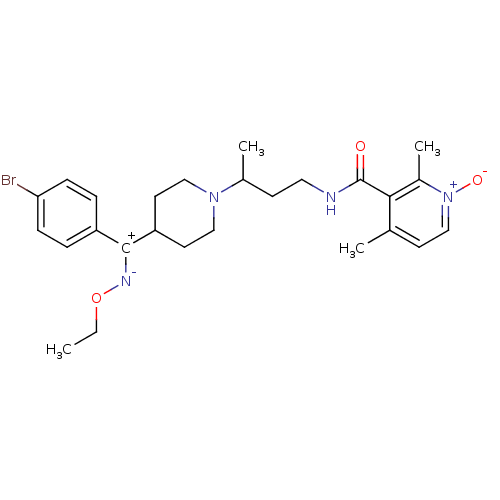
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; Langille, J; Di Fluri, M; Bogucki, D; Yang, W; Li, T; Wang, L; Nan, S; Baird, I; Metz, M; Darkes, M; Labrecque, J; Lau, G; Fricker, S; Huskens, D; Schols, D Design and synthesis of pyridin-2-yloxymethylpiperidin-1-ylbutyl amide CCR5 antagonists that are potent inhibitors of M-tropic (R5) HIV-1 replication. Bioorg Med Chem Lett21:2450-5 (2011) [PubMed] Article
Skerlj, R; Bridger, G; Zhou, Y; Bourque, E; Langille, J; Di Fluri, M; Bogucki, D; Yang, W; Li, T; Wang, L; Nan, S; Baird, I; Metz, M; Darkes, M; Labrecque, J; Lau, G; Fricker, S; Huskens, D; Schols, D Design and synthesis of pyridin-2-yloxymethylpiperidin-1-ylbutyl amide CCR5 antagonists that are potent inhibitors of M-tropic (R5) HIV-1 replication. Bioorg Med Chem Lett21:2450-5 (2011) [PubMed] Article