

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250661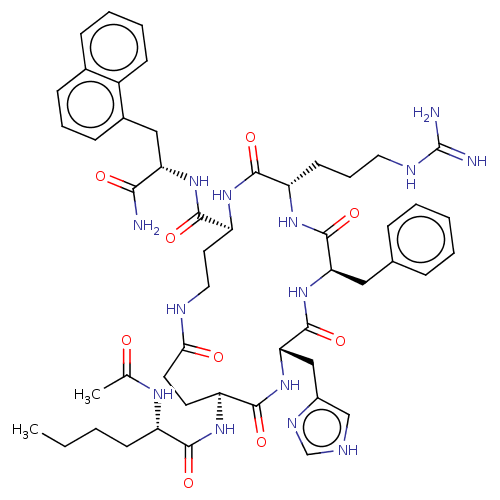 (US9447148, 9.30) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.00500 | -67.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250652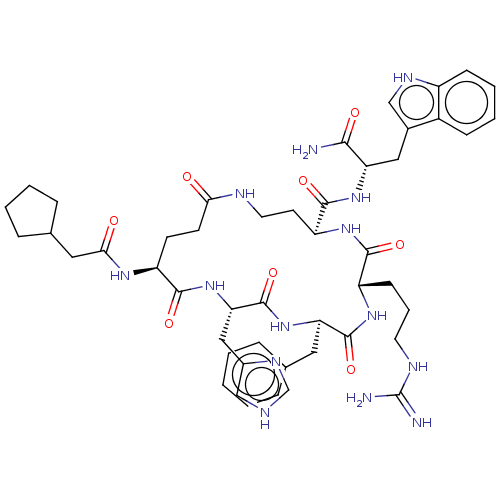 (US9447148, 9.21) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250653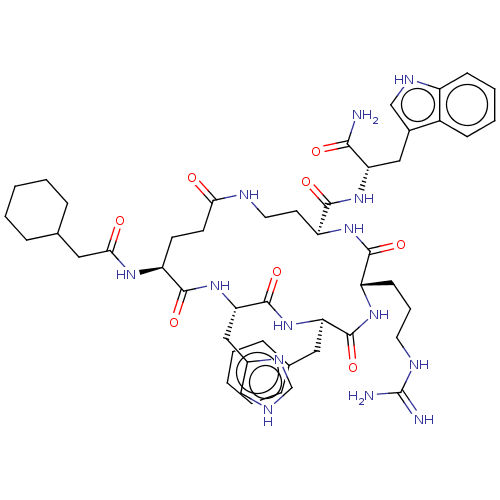 (US9447148, 9.22) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250642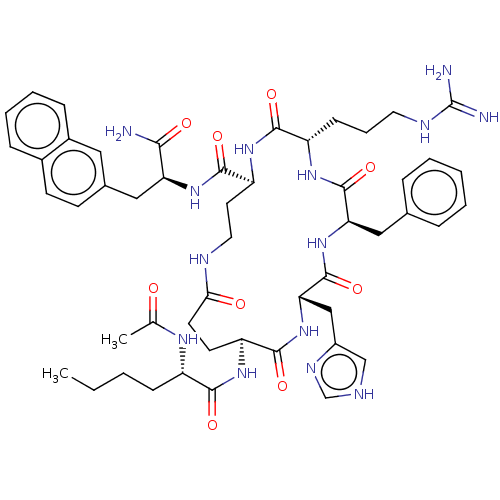 (US9447148, 9.10) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250633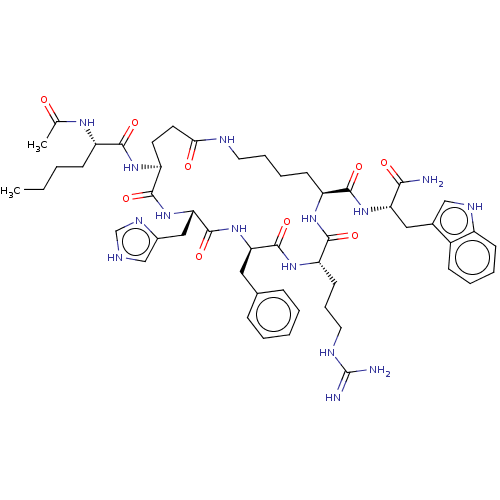 (US9447148, 9.1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250634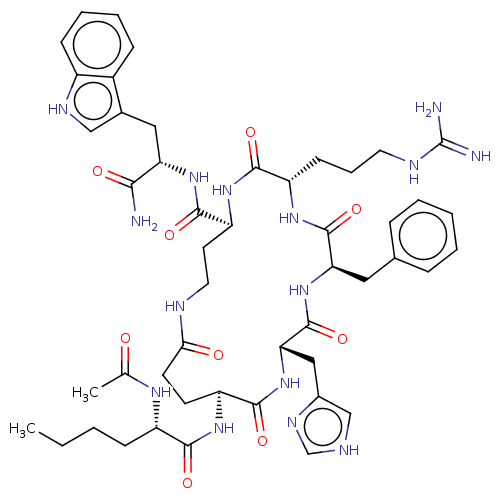 (US9447148, 9.2 | US9447148, 9.31) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | -64.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250668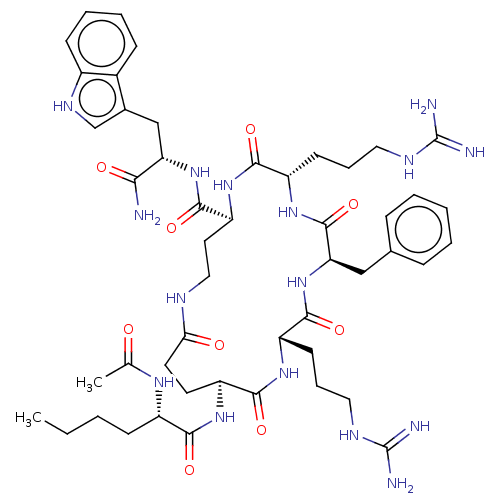 (US9447148, 9.37 | US9447148, 9.50) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | -64.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250668 (US9447148, 9.37 | US9447148, 9.50) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | -64.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250649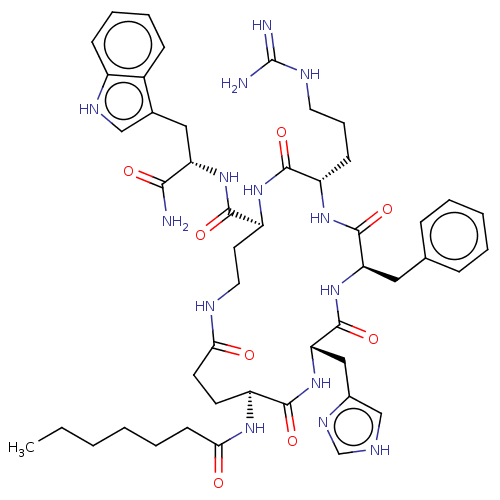 (US9447148, 9.18) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250673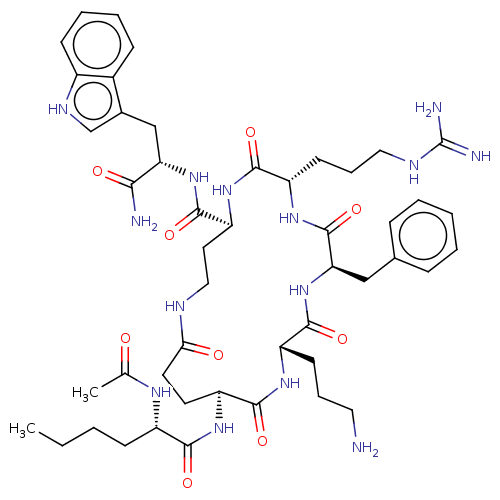 (US9447148, 9.42 | US9447148, 9.55) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250658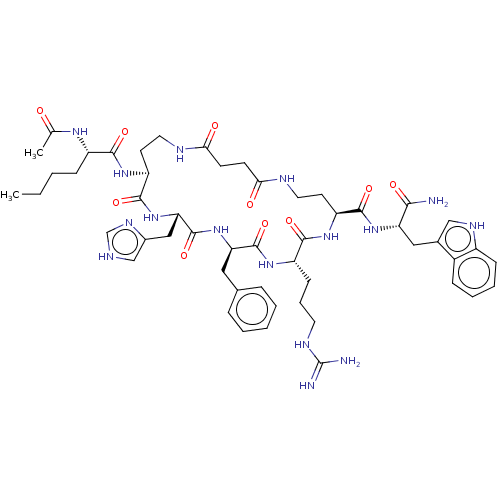 (US9447148, 9.27) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250673 (US9447148, 9.42 | US9447148, 9.55) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | -64.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250643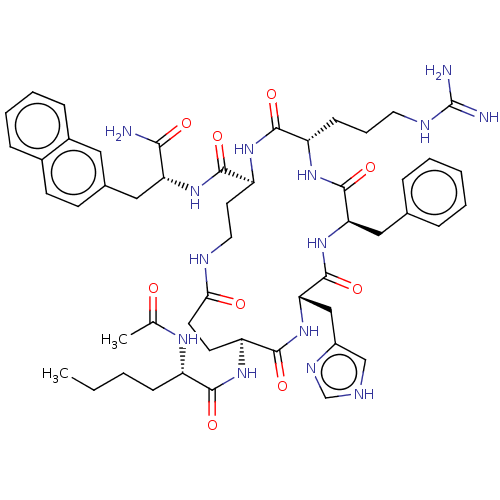 (US9447148, 9.11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | -63.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250651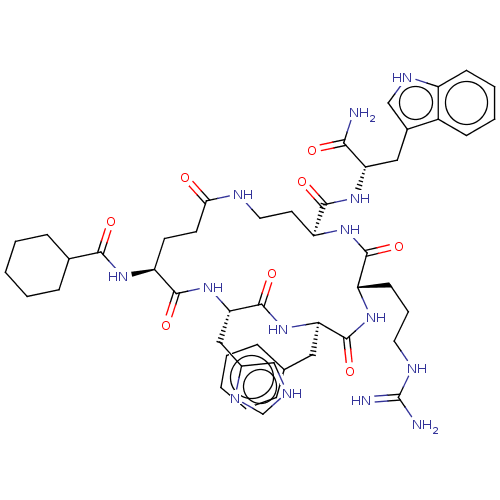 (US9447148, 9.20) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | -62.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250660 (US9447148, 9.29) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | -62.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250648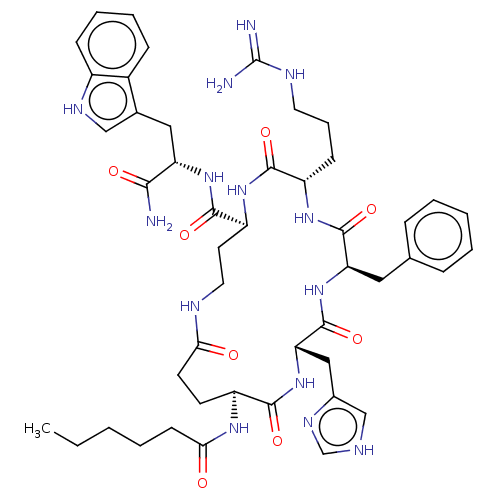 (US9447148, 9.17) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | -62.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241371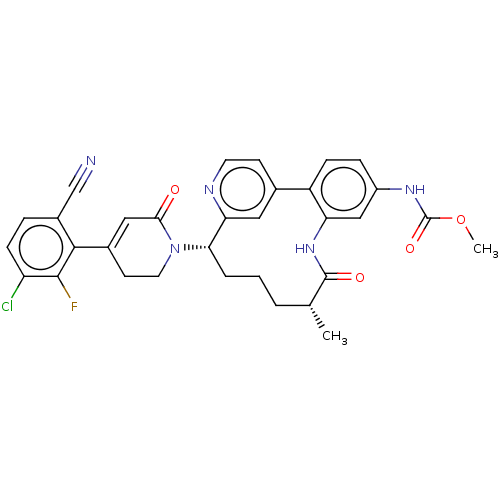 (US9409908, 3 | US9951071, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241371 (US9409908, 3 | US9951071, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230326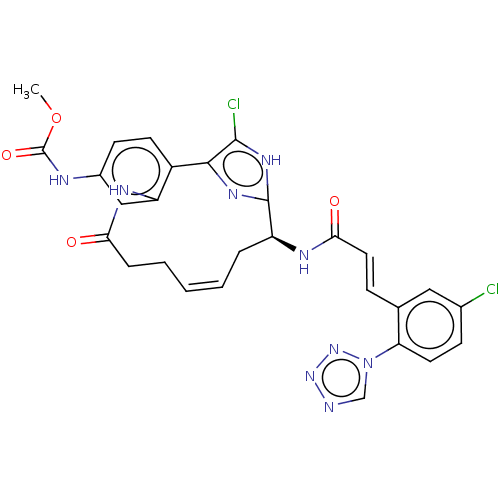 (CHEMBL4060950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250659 (US9447148, 9.28) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250655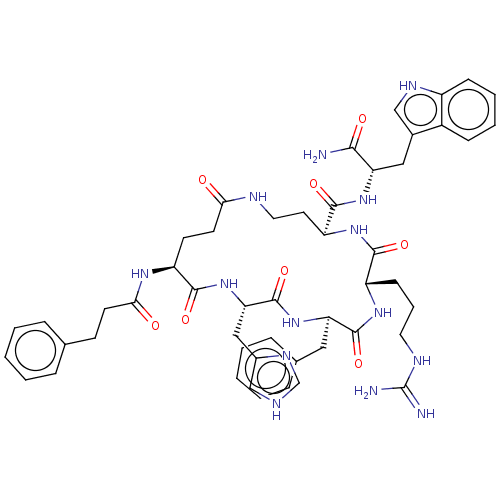 (US9447148, 9.24) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241399 (US9409908, 31 | US9951071, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250635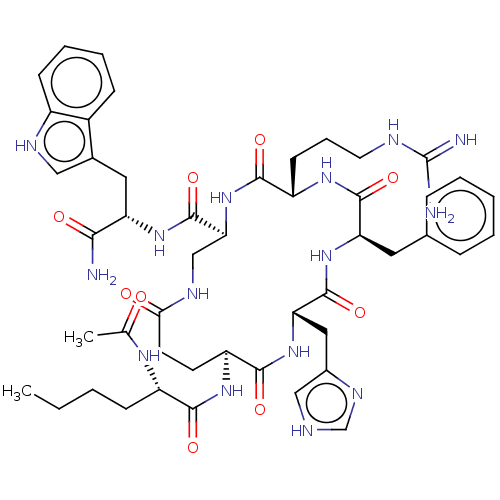 (US9447148, 9.3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241399 (US9409908, 31 | US9951071, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250671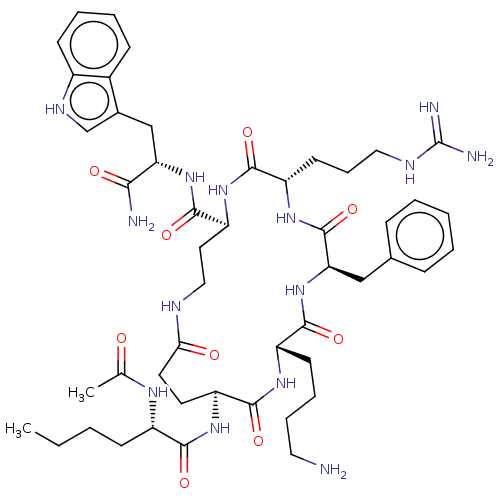 (US9447148, 9.40 | US9447148, 9.53) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250671 (US9447148, 9.40 | US9447148, 9.53) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250654 (US9447148, 9.23) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241503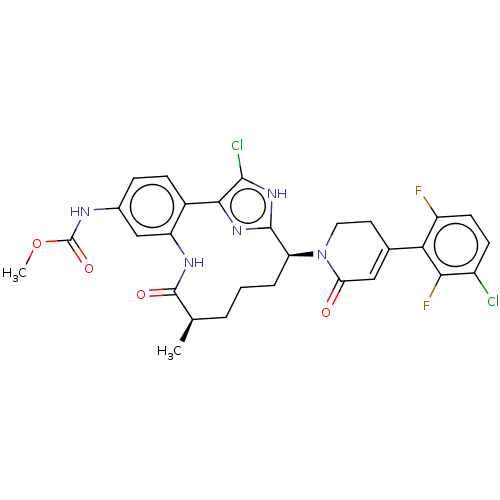 (US9409908, 135 | US9951071, Example 135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241552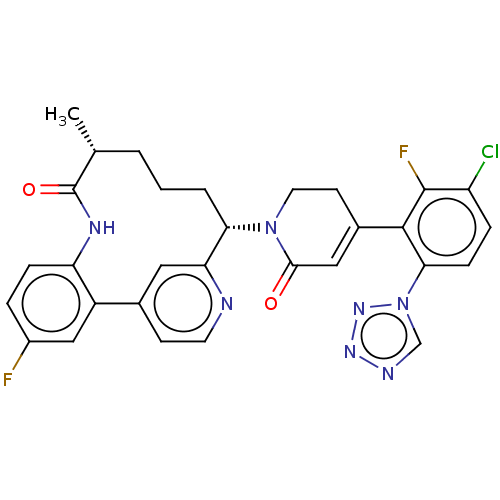 (US9409908, 184 | US9951071, Example 184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250672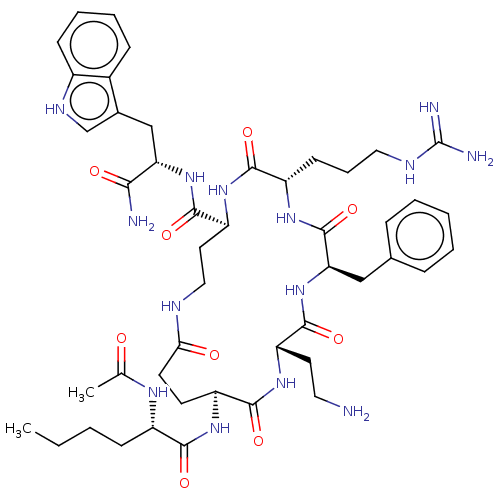 (US9447148, 9.41 | US9447148, 9.54) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | -61.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241552 (US9409908, 184 | US9951071, Example 184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241566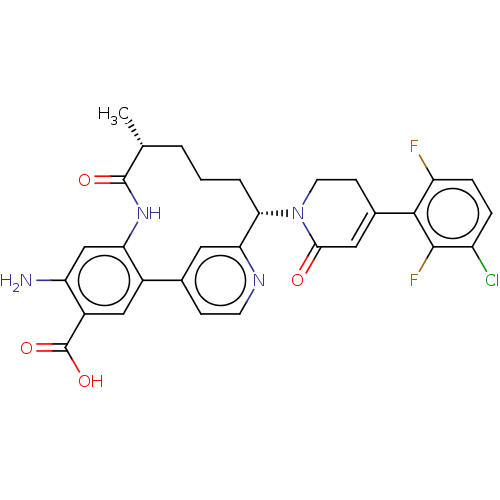 (US9409908, 198 | US9951071, Example 198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241567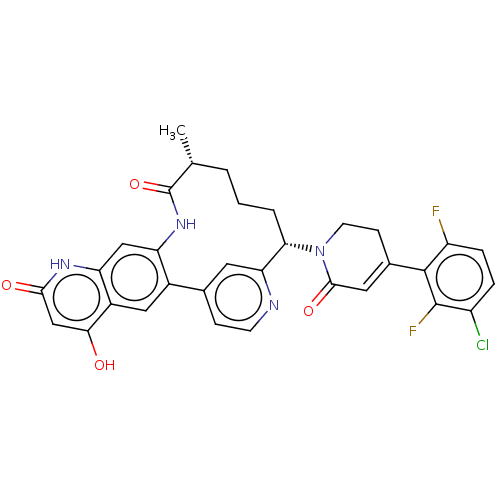 (US9409908, 199 | US9951071, Example 199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241436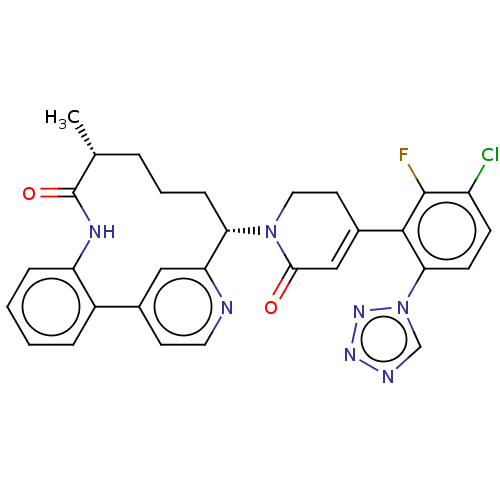 (US9409908, 68 | US9951071, Example 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241436 (US9409908, 68 | US9951071, Example 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250672 (US9447148, 9.41 | US9447148, 9.54) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | -61.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241566 (US9409908, 198 | US9951071, Example 198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241567 (US9409908, 199 | US9951071, Example 199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241503 (US9409908, 135 | US9951071, Example 135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160159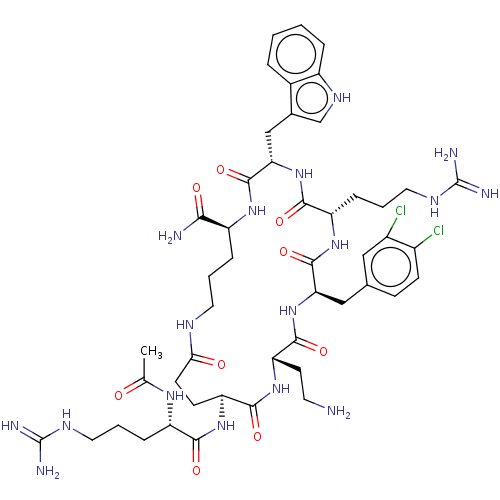 (US9040663, 5) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0550 | -60.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160158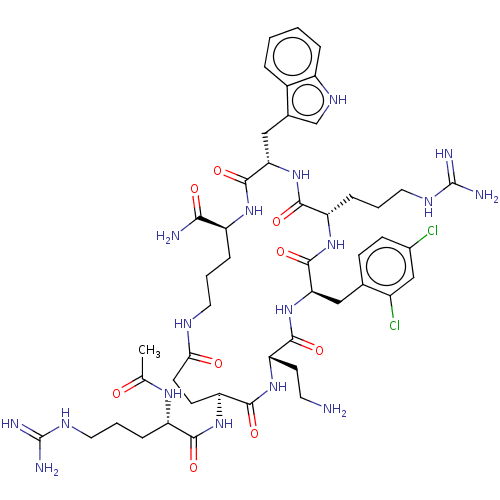 (US9040663, 4) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0550 | -60.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM160174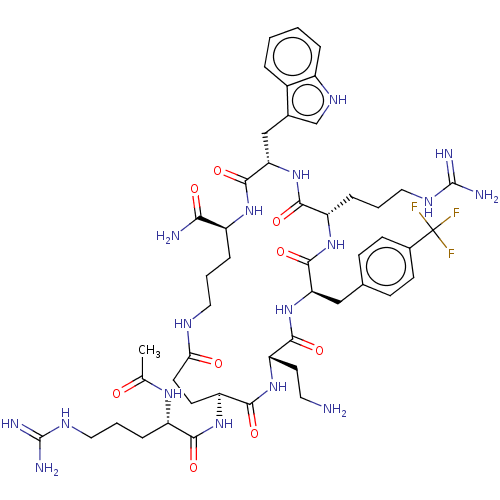 (US9040663, 20) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0600 | -60.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
AstraZeneca AB US Patent | Assay Description A competitive inhibition binding assay was performed for exemplified peptides according to the invention using membrane homogenates prepared from HEK... | US Patent US9040663 (2015) BindingDB Entry DOI: 10.7270/Q29885SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241451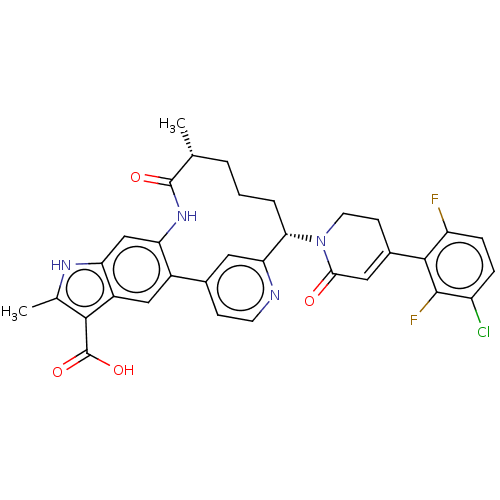 (US9409908, 83 | US9951071, Example 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241400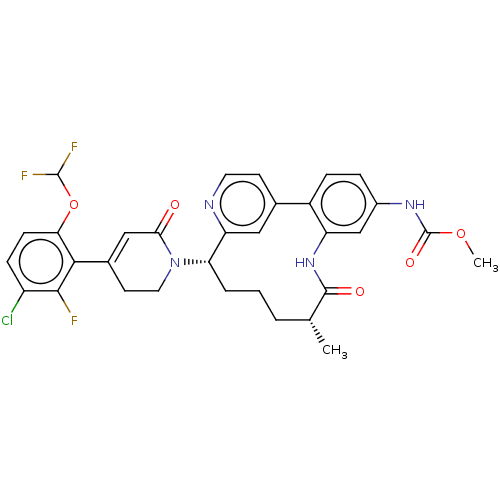 (US9409908, 32 | US9951071, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241400 (US9409908, 32 | US9951071, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50364330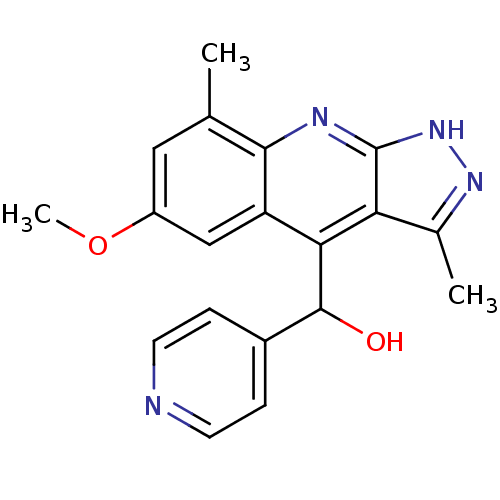 (CHEMBL1949936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 1335-9 (2012) Article DOI: 10.1016/j.bmcl.2011.12.080 BindingDB Entry DOI: 10.7270/Q2RF5VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50269207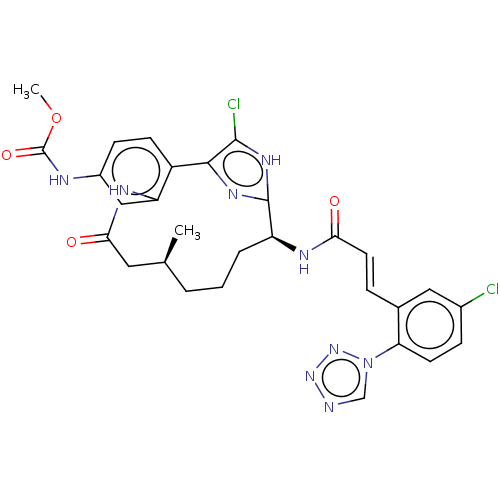 (CHEMBL4097522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 27: 3833-3839 (2017) Article DOI: 10.1016/j.bmcl.2017.06.058 BindingDB Entry DOI: 10.7270/Q2GT5QPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241451 (US9409908, 83 | US9951071, Example 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM250674 (US9447148, 9.43) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0650 | -60.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Palatin Technologies, Inc. US Patent | Assay Description A competitive inhibition binding assay was performed using membrane homogenates prepared from HEK-293 cells that express recombinant hMCR-1a or hMCR-... | US Patent US9447148 (2016) BindingDB Entry DOI: 10.7270/Q2RJ4HD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241583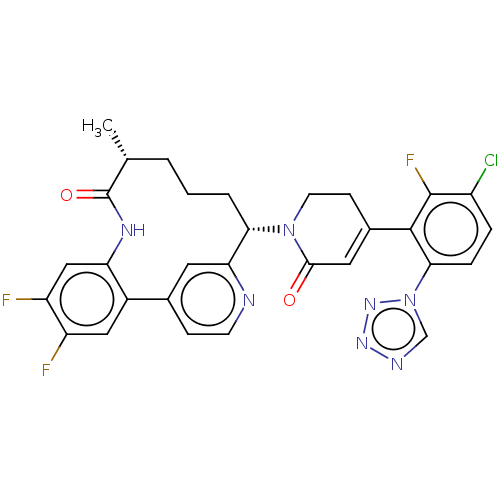 (US9409908, 215 | US9951071, Example 215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8479 total ) | Next | Last >> |