 Found 57566 hits with Last Name = 'li' and Initial = 't'
Found 57566 hits with Last Name = 'li' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059510

(8-Methoxy-3-phenyl-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C19H17NO3/c1-22-14-7-8-16-15-9-10-20(13-5-3-2-4-6-13)12-17(15)19(21)23-18(16)11-14/h2-8,11H,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50030745
(CHEMBL3342185 | acs.jmedchem.1c00409_ST.412)Show SMILES CC(C)(C)OC(=O)NN(CC(=O)N(Cc1ccccc1)Cc1ccccc1)C#N Show InChI InChI=1S/C22H26N4O3/c1-22(2,3)29-21(28)24-26(17-23)16-20(27)25(14-18-10-6-4-7-11-18)15-19-12-8-5-9-13-19/h4-13H,14-16H2,1-3H3,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50030746
(CHEMBL3342184 | acs.jmedchem.1c00409_ST.413)Show InChI InChI=1S/C16H22N4O3/c1-16(2,3)23-15(22)18-20(12-17)11-14(21)19(4)10-13-8-6-5-7-9-13/h5-9H,10-11H2,1-4H3,(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50184069
(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]CGRP from human cloned CLR/RAMP1 receptor expressed in E10 cells |
Bioorg Med Chem Lett 16: 2595-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.051
BindingDB Entry DOI: 10.7270/Q2HT2NX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50008834
(CHEMBL3236671)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C34H52N8O6/c1-19-9-7-10-20(2)25(19)18-29(32(46)41-28(33(47)48)11-5-6-13-35)42-31(45)27(12-8-14-39-34(37)38)40-30(44)26(36)17-24-21(3)15-23(43)16-22(24)4/h7,9-10,15-16,26-29,43H,5-6,8,11-14,17-18,35-36H2,1-4H3,(H,40,44)(H,41,46)(H,42,45)(H,47,48)(H4,37,38,39)/t26-,27+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00935 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes after 2 hrs |
Bioorg Med Chem 22: 2333-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.011
BindingDB Entry DOI: 10.7270/Q20P11JG |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356281
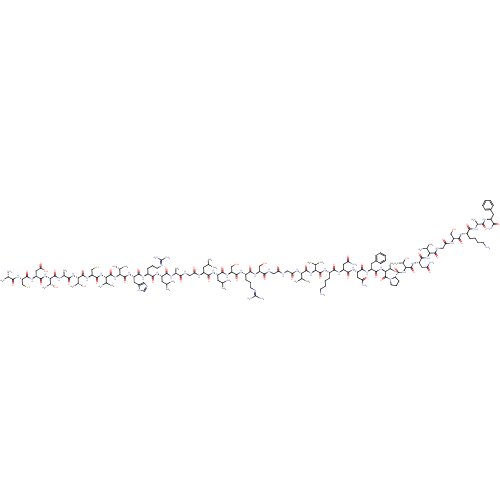
(CHEMBL1910953)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C163H269N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,62,72-88,92-111,120-128,215-221,264-265H,31-37,42-61,63-71,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,175,182)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177)/t81-,82-,83-,84-,85+,86+,87+,88+,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-,124-,125-,126-,127-,128-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(GUINEA PIG) | BDBM50590649
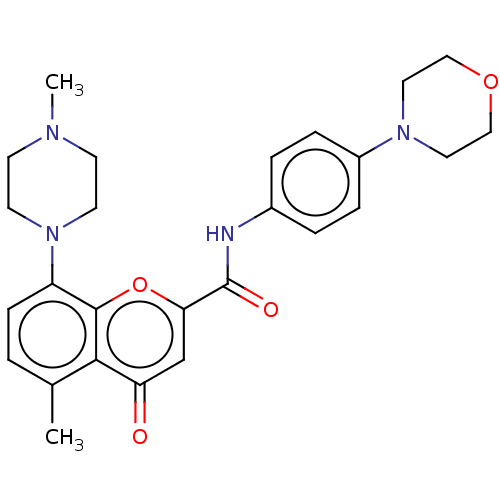
(CHEMBL5209320)Show SMILES Cc1ccc(N2CCN([11CH3])CC2)c2oc(cc(=O)c12)C(=O)Nc1ccc(cc1)N1CCOCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356281

(CHEMBL1910953)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C163H269N51O49S2/c1-73(2)52-97(186-116(226)65-179-131(233)82(18)183-139(241)98(53-74(3)4)193-137(239)94(44-35-49-176-162(171)172)188-142(244)101(57-91-62-175-72-182-91)199-159(261)128(88(24)221)213-156(258)123(79(13)14)207-151(253)110(71-265)204-160(262)126(86(22)219)210-133(235)84(20)185-157(259)125(85(21)218)211-147(249)105(61-119(229)230)198-150(252)109(70-264)203-130(232)81(17)166)140(242)194-99(54-75(5)6)141(243)202-108(69-217)149(251)190-95(45-36-50-177-163(173)174)138(240)201-106(67-215)134(236)180-63-115(225)178-64-118(228)205-121(77(9)10)155(257)208-122(78(11)12)154(256)191-93(43-32-34-48-165)136(238)196-102(58-112(167)222)144(246)197-103(59-113(168)223)143(245)195-100(56-90-40-29-26-30-41-90)145(247)209-124(80(15)16)161(263)214-51-37-46-111(214)152(254)212-127(87(23)220)158(260)200-104(60-114(169)224)146(248)206-120(76(7)8)153(255)181-66-117(227)187-107(68-216)148(250)189-92(42-31-33-47-164)135(237)184-83(19)132(234)192-96(129(170)231)55-89-38-27-25-28-39-89/h25-30,38-41,62,72-88,92-111,120-128,215-221,264-265H,31-37,42-61,63-71,164-166H2,1-24H3,(H2,167,222)(H2,168,223)(H2,169,224)(H2,170,231)(H,175,182)(H,178,225)(H,179,233)(H,180,236)(H,181,255)(H,183,241)(H,184,237)(H,185,259)(H,186,226)(H,187,227)(H,188,244)(H,189,250)(H,190,251)(H,191,256)(H,192,234)(H,193,239)(H,194,242)(H,195,245)(H,196,238)(H,197,246)(H,198,252)(H,199,261)(H,200,260)(H,201,240)(H,202,243)(H,203,232)(H,204,262)(H,205,228)(H,206,248)(H,207,253)(H,208,257)(H,209,247)(H,210,235)(H,211,249)(H,212,254)(H,213,258)(H,229,230)(H4,171,172,176)(H4,173,174,177)/t81-,82-,83-,84-,85+,86+,87+,88+,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,120-,121-,122-,123-,124-,125-,126-,127-,128-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay in the absence of MgCl2 |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50030747
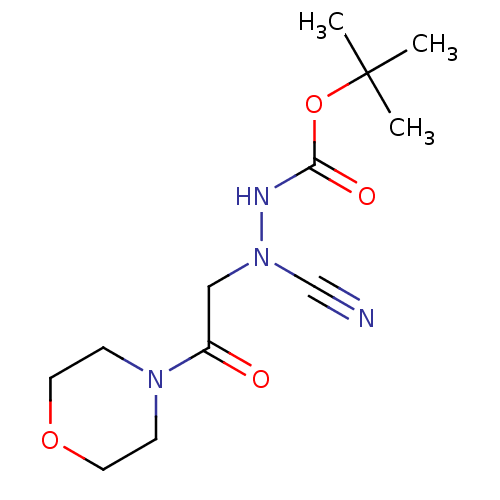
(CHEMBL3342183)Show InChI InChI=1S/C12H20N4O4/c1-12(2,3)20-11(18)14-16(9-13)8-10(17)15-4-6-19-7-5-15/h4-8H2,1-3H3,(H,14,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins |
ACS Med Chem Lett 5: 1076-81 (2014)
Article DOI: 10.1021/ml500238q
BindingDB Entry DOI: 10.7270/Q20P11NT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271367
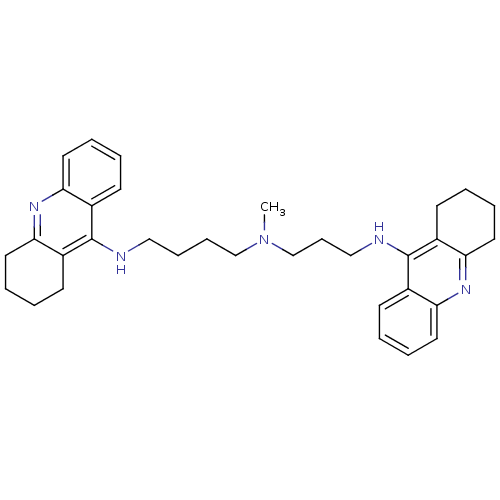
(CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H43N5/c1-39(24-12-22-36-34-27-15-4-8-19-31(27)38-32-20-9-5-16-28(32)34)23-11-10-21-35-33-25-13-2-6-17-29(25)37-30-18-7-3-14-26(30)33/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50184069

(CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](Cc1cc(Br)c(O)c(Br)c1)NC(=O)N1CCC(CC1)N1Cc2ccccc2NC1=O)C(=O)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C38H47Br2N9O5/c39-29-21-25(22-30(40)34(29)50)23-33(45-37(53)48-15-10-28(11-16-48)49-24-26-5-1-2-6-31(26)44-38(49)54)35(51)43-32(7-3-4-12-41)36(52)47-19-17-46(18-20-47)27-8-13-42-14-9-27/h1-2,5-6,8-9,13-14,21-22,28,32-33,50H,3-4,7,10-12,15-20,23-24,41H2,(H,43,51)(H,44,54)(H,45,53)/t32-,33+/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50271556
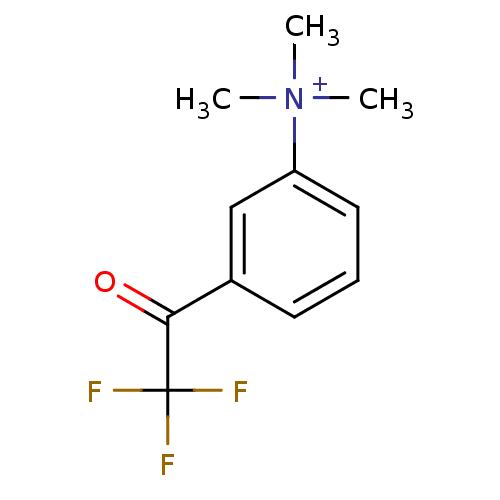
(CHEMBL525622 | N,N,N-trimethyl-3-(2,2,2-trifluoroa...)Show InChI InChI=1S/C11H13F3NO/c1-15(2,3)9-6-4-5-8(7-9)10(16)11(12,13)14/h4-7H,1-3H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007522
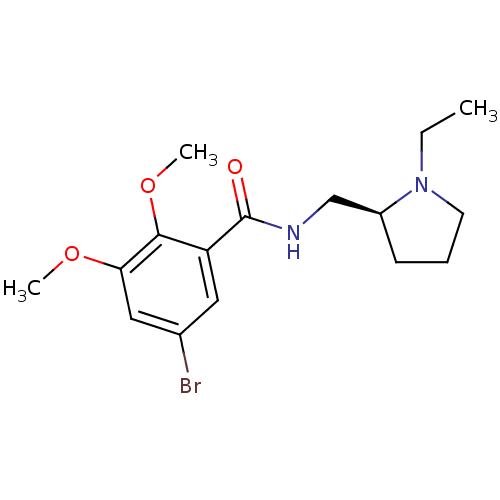
(5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...)Show InChI InChI=1S/C16H23BrN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 |
J Med Chem 34: 948-55 (1991)
BindingDB Entry DOI: 10.7270/Q2GT5NS9 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50063292
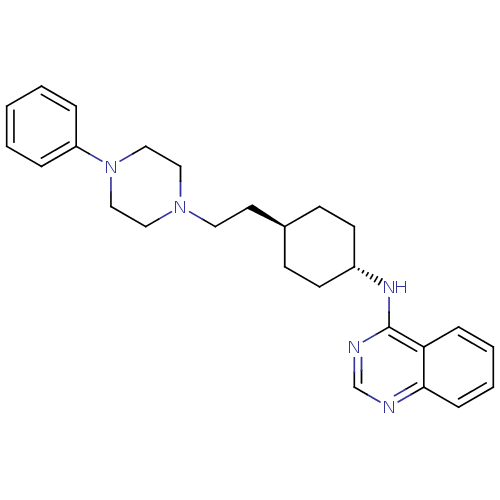
(CHEMBL349426 | {4-[2-(4-Phenyl-piperazin-1-yl)-eth...)Show SMILES C(CN1CCN(CC1)c1ccccc1)[C@H]1CC[C@@H](CC1)Nc1ncnc2ccccc12 |wU:17.22,wD:14.15,(10.31,-6.02,;11.15,-7.32,;12.69,-7.24,;13.39,-5.86,;14.93,-5.79,;15.76,-7.07,;15.07,-8.45,;13.53,-8.53,;17.3,-7,;17.99,-5.63,;19.5,-5.53,;20.36,-6.83,;19.66,-8.19,;18.13,-8.28,;8.79,-6.11,;7.95,-4.81,;6.42,-4.88,;5.72,-6.26,;6.55,-7.54,;8.09,-7.47,;4.18,-6.33,;3.34,-5.04,;4.04,-3.66,;3.23,-2.36,;1.69,-2.43,;.99,-3.81,;-.55,-3.87,;-1.26,-5.23,;-.44,-6.54,;1.1,-6.47,;1.8,-5.11,)| Show InChI InChI=1S/C26H33N5/c1-2-6-23(7-3-1)31-18-16-30(17-19-31)15-14-21-10-12-22(13-11-21)29-26-24-8-4-5-9-25(24)27-20-28-26/h1-9,20-22H,10-19H2,(H,27,28,29)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells |
J Med Chem 41: 760-71 (1998)
Article DOI: 10.1021/jm9707378
BindingDB Entry DOI: 10.7270/Q20G3J97 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50290221
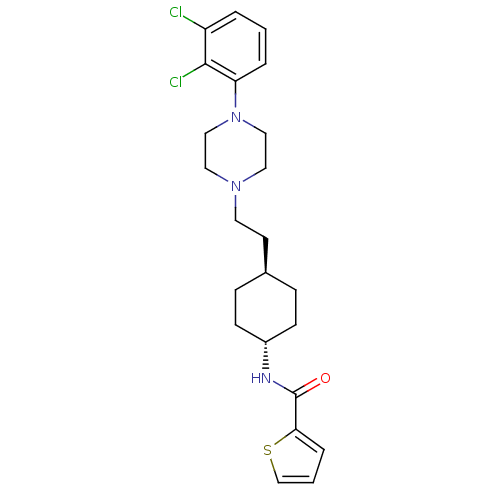
(CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...)Show SMILES Clc1cccc(N2CCN(CC[C@H]3CC[C@@H](CC3)NC(=O)c3cccs3)CC2)c1Cl |wU:12.11,wD:15.18,(20.53,-4.53,;19.79,-5.88,;20.6,-7.19,;19.85,-8.55,;18.31,-8.59,;17.52,-7.26,;15.98,-7.29,;15.24,-8.63,;13.7,-8.66,;12.91,-7.36,;11.37,-7.38,;10.6,-8.73,;9.06,-8.73,;8.29,-10.06,;6.75,-10.06,;5.98,-8.73,;6.74,-7.4,;8.28,-7.4,;4.44,-8.75,;3.67,-10.08,;2.13,-10.09,;4.44,-11.41,;5.98,-11.49,;5.83,-14.15,;4.3,-14.05,;3.59,-12.7,;13.66,-6,;15.2,-5.98,;18.25,-5.91,;17.46,-4.6,)| Show InChI InChI=1S/C23H29Cl2N3OS/c24-19-3-1-4-20(22(19)25)28-14-12-27(13-15-28)11-10-17-6-8-18(9-7-17)26-23(29)21-5-2-16-30-21/h1-5,16-18H,6-15H2,(H,26,29)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. |
Bioorg Med Chem Lett 7: 2403-2408 (1997)
Article DOI: 10.1016/S0960-894X(97)00443-5
BindingDB Entry DOI: 10.7270/Q27W6CQ0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50194487
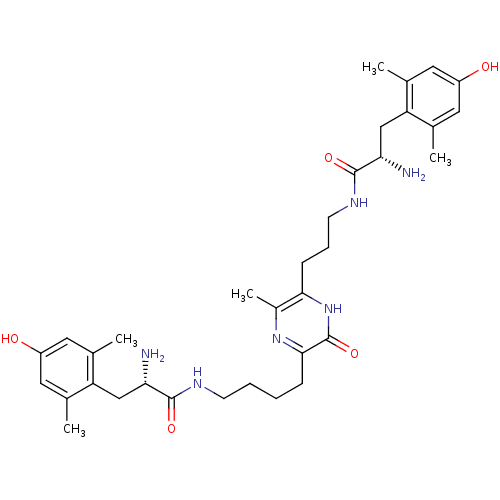
(3-[4'-(H-Dmt)-aminobutyl]-6-[3'-(H-Dmt)-aminopropy...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)NCCCCc1nc(C)c(CCCNC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)[nH]c1=O Show InChI InChI=1S/C34H48N6O5/c1-19-13-24(41)14-20(2)26(19)17-28(35)32(43)37-11-7-6-9-31-34(45)40-30(23(5)39-31)10-8-12-38-33(44)29(36)18-27-21(3)15-25(42)16-22(27)4/h13-16,28-29,41-42H,6-12,17-18,35-36H2,1-5H3,(H,37,43)(H,38,44)(H,40,45)/t28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat synaptosomes P2 fraction |
Bioorg Med Chem Lett 16: 5793-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.079
BindingDB Entry DOI: 10.7270/Q26Q1WWH |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50368060
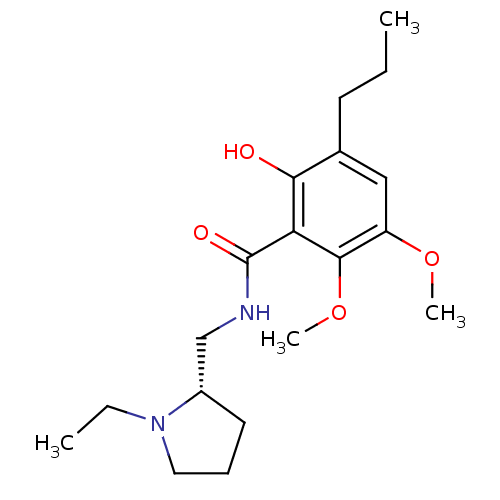
(CHEMBL1907695)Show SMILES CCCc1cc(OC)c(OC)c(C(=O)NC[C@@H]2CCCN2CC)c1O |r| Show InChI InChI=1S/C19H30N2O4/c1-5-8-13-11-15(24-3)18(25-4)16(17(13)22)19(23)20-12-14-9-7-10-21(14)6-2/h11,14,22H,5-10,12H2,1-4H3,(H,20,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 |
J Med Chem 33: 1155-63 (1990)
BindingDB Entry DOI: 10.7270/Q24X58DR |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50315401
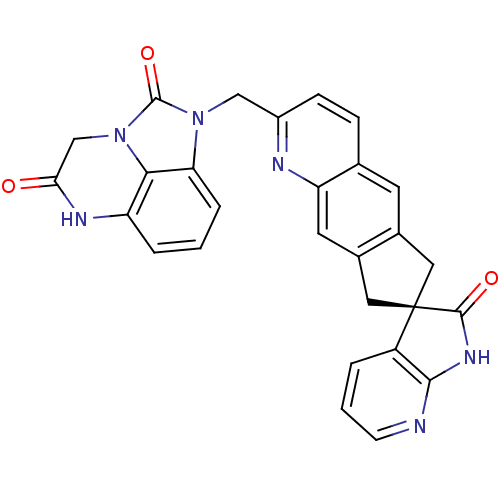
((R)-1-((2'-oxo-1',2',6,8-tetrahydrospiro[cyclopent...)Show SMILES O=C1Nc2ncccc2[C@]11Cc2cc3ccc(Cn4c5cccc6NC(=O)Cn(c56)c4=O)nc3cc2C1 |r| Show InChI InChI=1S/C28H20N6O3/c35-23-14-34-24-20(31-23)4-1-5-22(24)33(27(34)37)13-18-7-6-15-9-16-11-28(12-17(16)10-21(15)30-18)19-3-2-8-29-25(19)32-26(28)36/h1-10H,11-14H2,(H,31,35)(H,29,32,36)/t28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 20: 2572-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.086
BindingDB Entry DOI: 10.7270/Q2765FFZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50364856

(CHEMBL1950167)Show SMILES O=S1(=O)c2ccccc2-c2ccc(cc12)N1CCN2CCC1CC2 |(9.73,-11.78,;8.95,-13.11,;8.19,-11.77,;7.7,-14.01,;6.2,-13.68,;5.16,-14.83,;5.64,-16.29,;7.13,-16.62,;8.17,-15.48,;9.71,-15.48,;10.72,-16.64,;12.24,-16.34,;12.73,-14.89,;11.72,-13.72,;10.21,-14.03,;14.25,-14.59,;15.21,-15.86,;16.72,-15.87,;17.72,-14.73,;17.48,-13.28,;16.07,-12.5,;14.65,-13.09,;15.41,-14.42,;16.75,-13.64,)| Show InChI InChI=1S/C19H20N2O2S/c22-24(23)18-4-2-1-3-16(18)17-6-5-15(13-19(17)24)21-12-11-20-9-7-14(21)8-10-20/h1-6,13-14H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyllylcaconitine from alpha7 nAChR in rat brain |
Bioorg Med Chem Lett 22: 1633-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.126
BindingDB Entry DOI: 10.7270/Q2D21Z2W |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007508
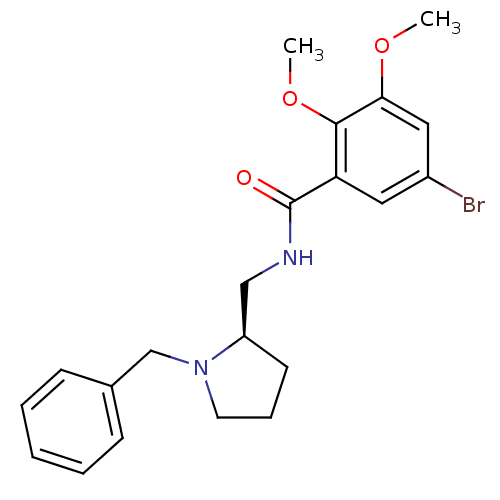
((R) N-(1-Benzyl-pyrrolidin-2-ylmethyl)-5-bromo-2,3...)Show SMILES COc1cc(Br)cc(C(=O)NC[C@H]2CCCN2Cc2ccccc2)c1OC Show InChI InChI=1S/C21H25BrN2O3/c1-26-19-12-16(22)11-18(20(19)27-2)21(25)23-13-17-9-6-10-24(17)14-15-7-4-3-5-8-15/h3-5,7-8,11-12,17H,6,9-10,13-14H2,1-2H3,(H,23,25)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 |
J Med Chem 34: 948-55 (1991)
BindingDB Entry DOI: 10.7270/Q2GT5NS9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide 1
(Homo sapiens (Human)) | BDBM50356282
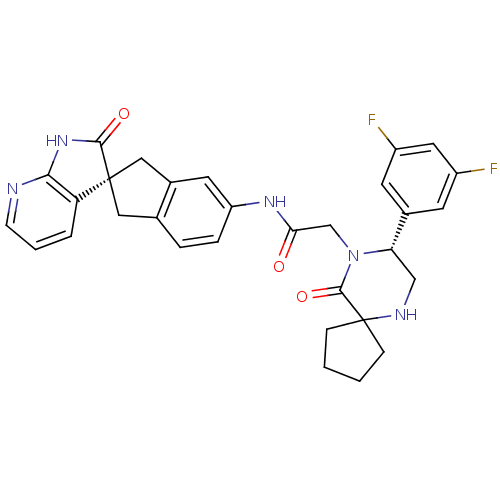
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]adrenomedullin form CGRP receptor in human SK-N-MC cell membrane by competitive binding assay |
Bioorg Med Chem Lett 21: 6705-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.056
BindingDB Entry DOI: 10.7270/Q2ZK5H25 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10597
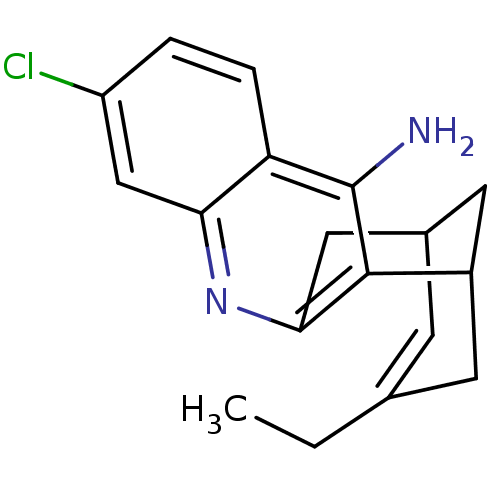
((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:11:9:5:3.2.7,19:8:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... |
J Med Chem 49: 3421-5 (2006)
Article DOI: 10.1021/jm060257t
BindingDB Entry DOI: 10.7270/Q2WW7FWB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity against Rat 5-hydroxytryptamine 7 receptor using [3H]-5-HT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting |
J Med Chem 58: 2609-22 (2015)
Article DOI: 10.1021/jm501915g
BindingDB Entry DOI: 10.7270/Q2N29ZNQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50368067
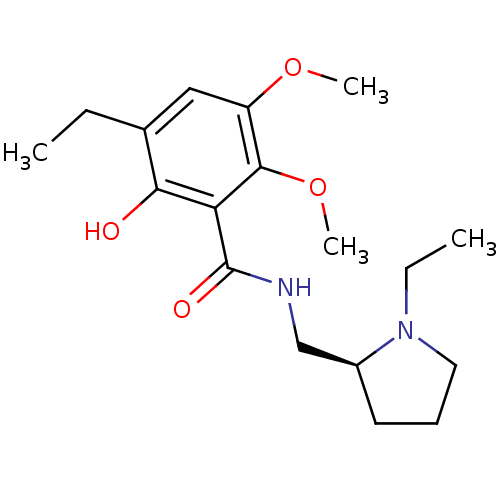
(CHEMBL1907702)Show SMILES CCN1CCC[C@H]1CNC(=O)c1c(O)c(CC)cc(OC)c1OC |r| Show InChI InChI=1S/C18H28N2O4/c1-5-12-10-14(23-3)17(24-4)15(16(12)21)18(22)19-11-13-8-7-9-20(13)6-2/h10,13,21H,5-9,11H2,1-4H3,(H,19,22)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 |
J Med Chem 33: 1155-63 (1990)
BindingDB Entry DOI: 10.7270/Q24X58DR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071565
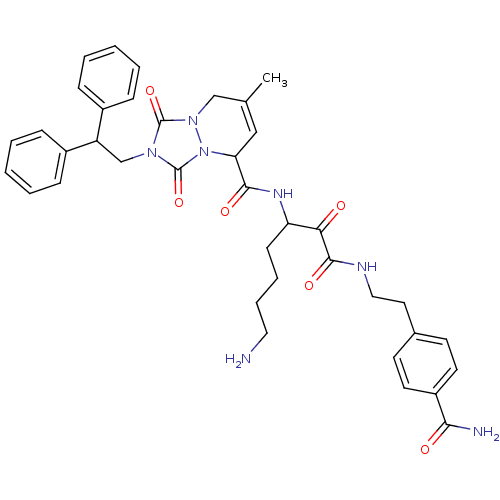
(2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...)Show SMILES CC1=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc2ccc(cc2)C(N)=O)n2n(C1)c(=O)n(CC(c1ccccc1)c1ccccc1)c2=O |t:1| Show InChI InChI=1S/C38H43N7O6/c1-25-22-32(35(48)42-31(14-8-9-20-39)33(46)36(49)41-21-19-26-15-17-29(18-16-26)34(40)47)45-38(51)43(37(50)44(45)23-25)24-30(27-10-4-2-5-11-27)28-12-6-3-7-13-28/h2-7,10-13,15-18,22,30-32H,8-9,14,19-21,23-24,39H2,1H3,(H2,40,47)(H,41,49)(H,42,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50279404
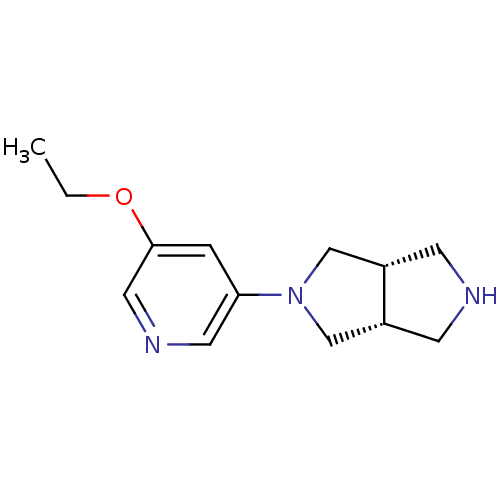
(CHEMBL447388 | cis-2-(5-Ethoxy-3-pyridinyl)octahyd...)Show InChI InChI=1S/C13H19N3O/c1-2-17-13-3-12(6-15-7-13)16-8-10-4-14-5-11(10)9-16/h3,6-7,10-11,14H,2,4-5,8-9H2,1H3/t10-,11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane |
J Med Chem 52: 4126-41 (2009)
Article DOI: 10.1021/jm900249k
BindingDB Entry DOI: 10.7270/Q2GT5P3R |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342639
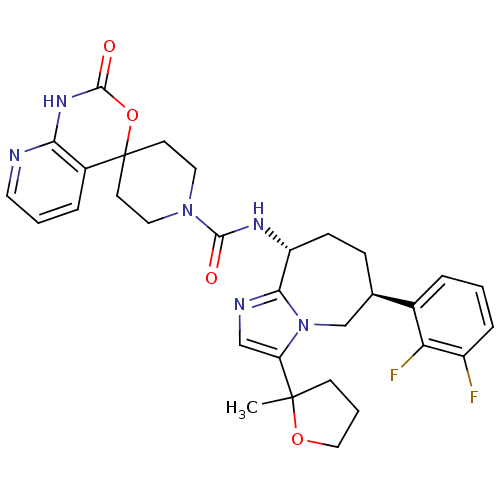
(CHEMBL1770729 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC1(CCCO1)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H34F2N6O4/c1-30(10-4-16-42-30)24-17-35-27-23(9-8-19(18-39(24)27)20-5-2-7-22(32)25(20)33)36-28(40)38-14-11-31(12-15-38)21-6-3-13-34-26(21)37-29(41)43-31/h2-3,5-7,13,17,19,23H,4,8-12,14-16,18H2,1H3,(H,36,40)(H,34,37,41)/t19-,23-,30?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342638
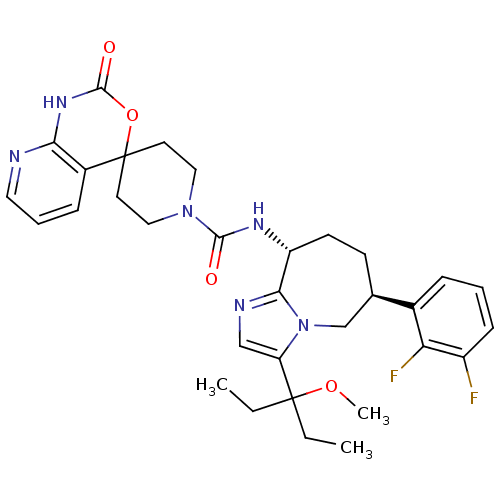
(CHEMBL1770728 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CCC(CC)(OC)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C32H38F2N6O4/c1-4-31(5-2,43-3)25-18-36-28-24(12-11-20(19-40(25)28)21-8-6-10-23(33)26(21)34)37-29(41)39-16-13-32(14-17-39)22-9-7-15-35-27(22)38-30(42)44-32/h6-10,15,18,20,24H,4-5,11-14,16-17,19H2,1-3H3,(H,37,41)(H,35,38,42)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445
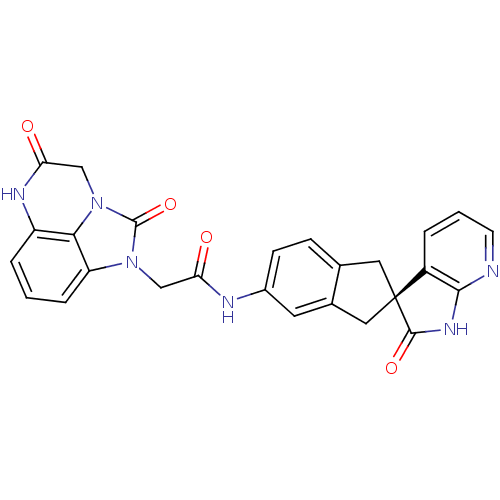
((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 19: 4740-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.057
BindingDB Entry DOI: 10.7270/Q2QV3MJH |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50220746
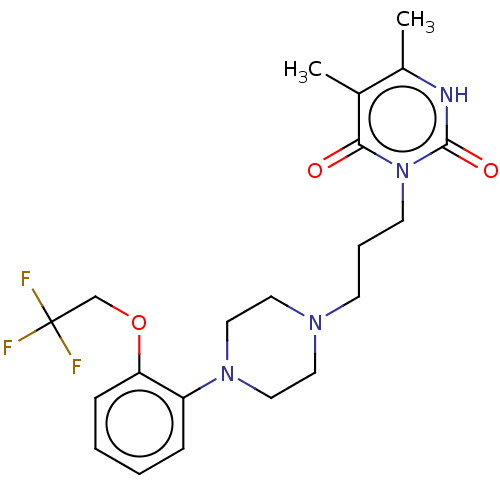
(CHEMBL292189)Show SMILES Cc1[nH]c(=O)n(CCCN2CCN(CC2)c2ccccc2OCC(F)(F)F)c(=O)c1C Show InChI InChI=1S/C21H27F3N4O3/c1-15-16(2)25-20(30)28(19(15)29)9-5-8-26-10-12-27(13-11-26)17-6-3-4-7-18(17)31-14-21(22,23)24/h3-4,6-7H,5,8-14H2,1-2H3,(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor |
Bioorg Med Chem Lett 13: 1873-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S46V50 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445

((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CLR from human CGRP expressed in HEK293 cells coexpressing human RAMP1 |
Bioorg Med Chem Lett 20: 2572-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.086
BindingDB Entry DOI: 10.7270/Q2765FFZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50472582

(CHEMBL45422)Show SMILES CNc1nc(CNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)ccc1C Show InChI InChI=1S/C21H25ClF2N4O/c1-14-3-5-16(27-19(14)25-2)12-26-13-21(24)7-9-28(10-8-21)20(29)15-4-6-18(23)17(22)11-15/h3-6,11,26H,7-10,12-13H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50254445

((S)-2-(2,5-dioxo-2,4,5,6-tetrahydro-1H-imidazo[1,5...)Show SMILES O=C(Cn1c2cccc3NC(=O)Cn(c23)c1=O)Nc1ccc2C[C@@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C26H20N6O4/c33-20(12-31-19-5-1-4-18-22(19)32(25(31)36)13-21(34)29-18)28-16-7-6-14-10-26(11-15(14)9-16)17-3-2-8-27-23(17)30-24(26)35/h1-9H,10-13H2,(H,28,33)(H,29,34)(H,27,30,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co
Curated by ChEMBL
| Assay Description
Displacement of [125I]hCGRP from human CGRP receptor expressed in HEK293 cells coexpressing RAMP1 |
Bioorg Med Chem Lett 19: 214-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.106
BindingDB Entry DOI: 10.7270/Q21C1XSK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50590640

(CHEMBL5177580)Show SMILES COc1ccccc1N1CCN(CCCCc2ccc3n(C)c(=O)oc3c2)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342625
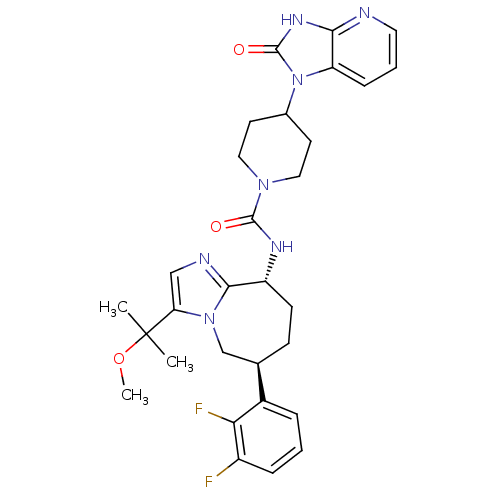
(CHEMBL1770715 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C30H35F2N7O3/c1-30(2,42-3)24-16-34-27-22(10-9-18(17-38(24)27)20-6-4-7-21(31)25(20)32)35-28(40)37-14-11-19(12-15-37)39-23-8-5-13-33-26(23)36-29(39)41/h4-8,13,16,18-19,22H,9-12,14-15,17H2,1-3H3,(H,35,40)(H,33,36,41)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50279405
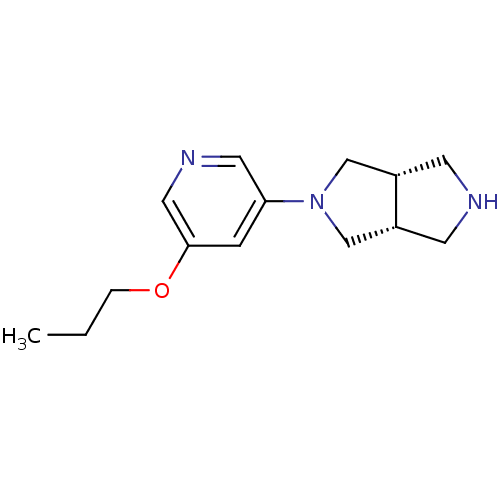
(CHEMBL488772 | cis-3-(5-Propyloxy-3-pyridinyl)-3,7...)Show InChI InChI=1S/C14H21N3O/c1-2-3-18-14-4-13(7-16-8-14)17-9-11-5-15-6-12(11)10-17/h4,7-8,11-12,15H,2-3,5-6,9-10H2,1H3/t11-,12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane |
J Med Chem 52: 4126-41 (2009)
Article DOI: 10.1021/jm900249k
BindingDB Entry DOI: 10.7270/Q2GT5P3R |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50221787
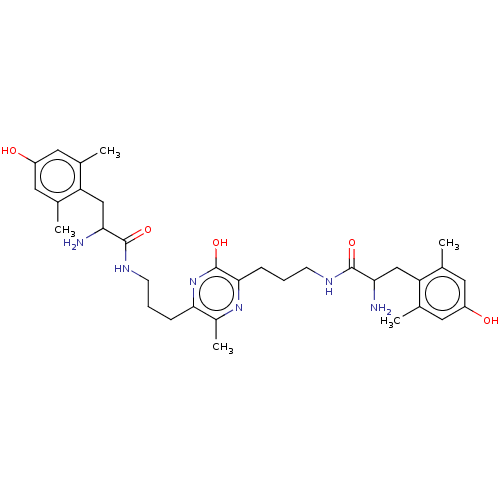
(CHEMBL3216418)Show SMILES Cl.Cl.Cc1cc(O)cc(C)c1CC(N)C(=O)NCCCc1nc(O)c(CCCNC(=O)C(N)Cc2c(C)cc(O)cc2C)nc1C Show InChI InChI=1S/C33H46N6O5.2ClH/c1-18-12-23(40)13-19(2)25(18)16-27(34)31(42)36-10-6-8-29-22(5)38-30(33(44)39-29)9-7-11-37-32(43)28(35)17-26-20(3)14-24(41)15-21(26)4;;/h12-15,27-28,40-41H,6-11,16-17,34-35H2,1-5H3,(H,36,42)(H,37,43)(H,39,44);2*1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Environmental Health Sciences
Curated by ChEMBL
| Assay Description
Compound was tested for Opioid receptor mu 1 agonism in isolated tissues from guinea pig ileum |
J Med Chem 47: 2599-610 (2004)
Article DOI: 10.1021/jm0304616
BindingDB Entry DOI: 10.7270/Q2RB755V |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50253328
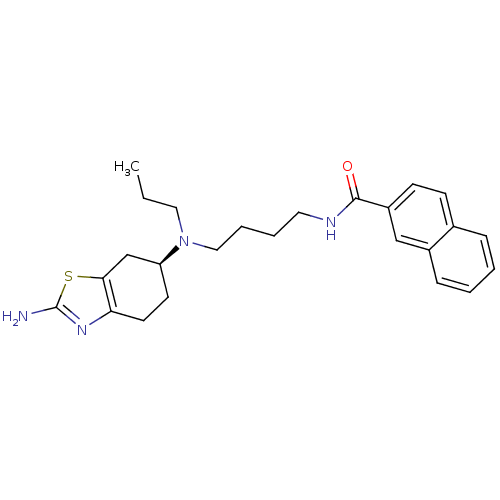
((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...)Show SMILES CCCN(CCCCNC(=O)c1ccc2ccccc2c1)[C@H]1CCc2nc(N)sc2C1 |r| Show InChI InChI=1S/C25H32N4OS/c1-2-14-29(21-11-12-22-23(17-21)31-25(26)28-22)15-6-5-13-27-24(30)20-10-9-18-7-3-4-8-19(18)16-20/h3-4,7-10,16,21H,2,5-6,11-15,17H2,1H3,(H2,26,28)(H,27,30)/t21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum |
J Med Chem 51: 5905-8 (2008)
Article DOI: 10.1021/jm800471h
BindingDB Entry DOI: 10.7270/Q2FN161H |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50288098
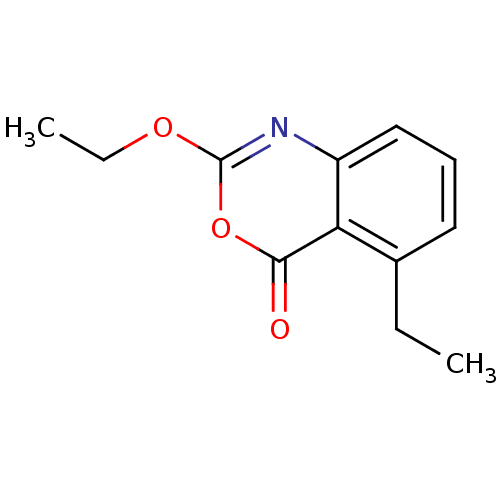
(2-Ethoxy-5-ethyl-benzo[d][1,3]oxazin-4-one | CHEMB...)Show InChI InChI=1S/C12H13NO3/c1-3-8-6-5-7-9-10(8)11(14)16-12(13-9)15-4-2/h5-7H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0430 | n/a | n/a | n/a | n/a | 0.0000145 | 3.39E+5 | n/a | n/a |
Syntex Research
Curated by ChEMBL
| Assay Description
Component deacylation rate constant for interaction with human leukocyte elastase |
J Med Chem 33: 464-79 (1990)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2PR7X6X |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50212613
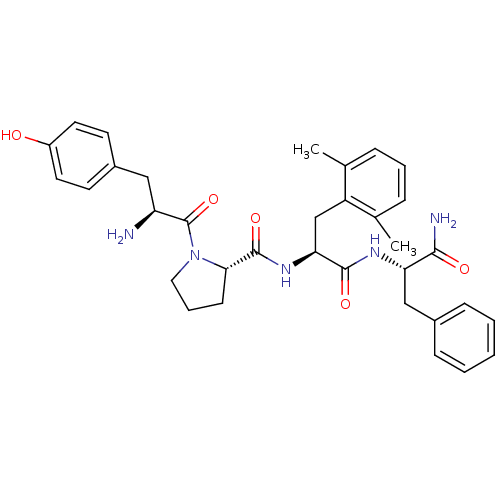
(CHEMBL266122 | Tyr-Pro-Dmp-Phe-NH2)Show SMILES Cc1cccc(C)c1C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H41N5O5/c1-21-8-6-9-22(2)26(21)20-29(32(42)37-28(31(36)41)19-23-10-4-3-5-11-23)38-33(43)30-12-7-17-39(30)34(44)27(35)18-24-13-15-25(40)16-14-24/h3-6,8-11,13-16,27-30,40H,7,12,17-20,35H2,1-2H3,(H2,36,41)(H,37,42)(H,38,43)/t27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosome membrane |
J Med Chem 50: 2753-66 (2007)
Article DOI: 10.1021/jm061238m
BindingDB Entry DOI: 10.7270/Q25B0264 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cytisine from alpha4beta2 nicotinic acetylcholine receptor in rat brain minus cerebellum membrane |
J Med Chem 52: 4126-41 (2009)
Article DOI: 10.1021/jm900249k
BindingDB Entry DOI: 10.7270/Q2GT5P3R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50327858
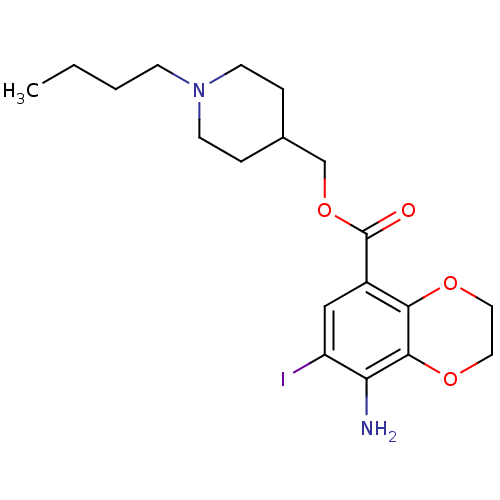
((1-Butylpiperidin-4-yl)methyl 8-amino-7-iodo-2,3-d...)Show InChI InChI=1S/C19H27IN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oslo
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 363: 146-60 (2001)
Article DOI: 10.1007/s002100000299
BindingDB Entry DOI: 10.7270/Q2222SBP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50288283
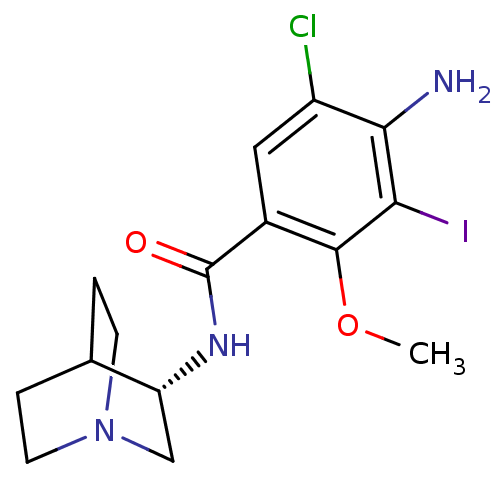
(4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chlor...)Show SMILES COc1c(I)c(N)c(Cl)cc1C(=O)N[C@@H]1CN2CCC1CC2 |wU:14.14,(11.77,-27.01,;11.77,-25.48,;10.45,-24.71,;9.1,-25.48,;9.1,-27.01,;7.78,-24.71,;6.45,-25.48,;7.78,-23.17,;6.45,-22.4,;9.1,-22.4,;10.45,-23.17,;11.77,-22.4,;11.77,-20.86,;13.1,-23.17,;14.43,-22.4,;15.77,-23.18,;17.09,-22.42,;17.11,-20.88,;15.77,-20.1,;14.43,-20.86,;15.24,-22.25,;16.38,-21.1,)| Show InChI InChI=1S/C15H19ClIN3O2/c1-22-14-9(6-10(16)13(18)12(14)17)15(21)19-11-7-20-4-2-8(11)3-5-20/h6,8,11H,2-5,7,18H2,1H3,(H,19,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 6: 2657-2662 (1996)
Article DOI: 10.1016/S0960-894X(96)00497-0
BindingDB Entry DOI: 10.7270/Q20V8CRP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data