| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Diacylglycerol O-acyltransferase 1 |
|---|
| Ligand | BDBM50341784 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_743559 (CHEMBL1768059) |
|---|
| IC50 | 44±n/a nM |
|---|
| Citation |  Qian, Y; Wertheimer, SJ; Ahmad, M; Cheung, AW; Firooznia, F; Hamilton, MM; Hayden, S; Li, S; Marcopulos, N; McDermott, L; Tan, J; Yun, W; Guo, L; Pamidimukkala, A; Chen, Y; Huang, KS; Ramsey, GB; Whittard, T; Conde-Knape, K; Taub, R; Rondinone, CM; Tilley, J; Bolin, D Discovery of orally active carboxylic acid derivatives of 2-phenyl-5-trifluoromethyloxazole-4-carboxamide as potent diacylglycerol acyltransferase-1 inhibitors for the potential treatment of obesity and diabetes. J Med Chem54:2433-46 (2011) [PubMed] Article Qian, Y; Wertheimer, SJ; Ahmad, M; Cheung, AW; Firooznia, F; Hamilton, MM; Hayden, S; Li, S; Marcopulos, N; McDermott, L; Tan, J; Yun, W; Guo, L; Pamidimukkala, A; Chen, Y; Huang, KS; Ramsey, GB; Whittard, T; Conde-Knape, K; Taub, R; Rondinone, CM; Tilley, J; Bolin, D Discovery of orally active carboxylic acid derivatives of 2-phenyl-5-trifluoromethyloxazole-4-carboxamide as potent diacylglycerol acyltransferase-1 inhibitors for the potential treatment of obesity and diabetes. J Med Chem54:2433-46 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Diacylglycerol O-acyltransferase 1 |
|---|
| Name: | Diacylglycerol O-acyltransferase 1 |
|---|
| Synonyms: | ACAT-related gene product 1 | AGRP1 | Acyl coA-diacylglycerol acyl transferase 1 (DGAT1) | DGAT | DGAT1 | DGAT1_HUMAN | Diacylglycerol Acyltransferase (DGAT1) | Diacylglycerol O-acyltransferase 1 | Diacylglycerol O-acyltransferase 1 (DGAT1) | Diglyceride acyltransferase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55297.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O75907 |
|---|
| Residue: | 488 |
|---|
| Sequence: | MGDRGSSRRRRTGSRPSSHGGGGPAAAEEEVRDAAAGPDVGAAGDAPAPAPNKDGDAGVG
SGHWELRCHRLQDSLFSSDSGFSNYRGILNWCVVMLILSNARLFLENLIKYGILVDPIQV
VSLFLKDPYSWPAPCLVIAANVFAVAAFQVEKRLAVGALTEQAGLLLHVANLATILCFPA
AVVLLVESITPVGSLLALMAHTILFLKLFSYRDVNSWCRRARAKAASAGKKASSAAAPHT
VSYPDNLTYRDLYYFLFAPTLCYELNFPRSPRIRKRFLLRRILEMLFFTQLQVGLIQQWM
VPTIQNSMKPFKDMDYSRIIERLLKLAVPNHLIWLIFFYWLFHSCLNAVAELMQFGDREF
YRDWWNSESVTYFWQNWNIPVHKWCIRHFYKPMLRRGSSKWMARTGVFLASAFFHEYLVS
VPLRMFRLWAFTGMMAQIPLAWFVGRFFQGNYGNAAVWLSLIIGQPIAVLMYVHDYYVLN
YEAPAAEA
|
|
|
|---|
| BDBM50341784 |
|---|
| n/a |
|---|
| Name | BDBM50341784 |
|---|
| Synonyms: | CHEMBL1766894 | N-(2-(2-phenyl-5-(trifluoromethyl)-1H-imidazole-4-carboxamido)ethyl)-6-(2,2,2-trifluoroethoxy)nicotinamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H17F6N5O3 |
|---|
| Mol. Mass. | 501.3818 |
|---|
| SMILES | FC(F)(F)COc1ccc(cn1)C(=O)NCCNC(=O)c1[nH]c(nc1C(F)(F)F)-c1ccccc1 |
|---|
| Structure | 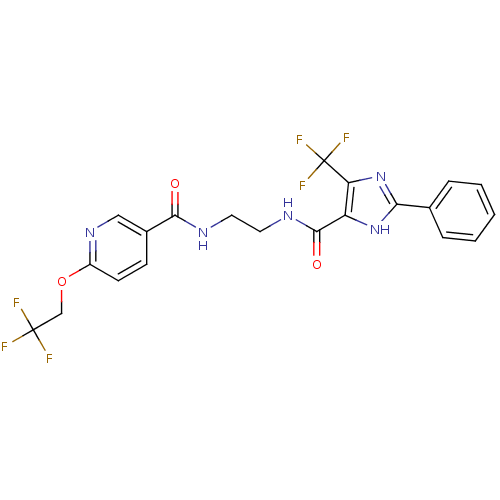
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Qian, Y; Wertheimer, SJ; Ahmad, M; Cheung, AW; Firooznia, F; Hamilton, MM; Hayden, S; Li, S; Marcopulos, N; McDermott, L; Tan, J; Yun, W; Guo, L; Pamidimukkala, A; Chen, Y; Huang, KS; Ramsey, GB; Whittard, T; Conde-Knape, K; Taub, R; Rondinone, CM; Tilley, J; Bolin, D Discovery of orally active carboxylic acid derivatives of 2-phenyl-5-trifluoromethyloxazole-4-carboxamide as potent diacylglycerol acyltransferase-1 inhibitors for the potential treatment of obesity and diabetes. J Med Chem54:2433-46 (2011) [PubMed] Article
Qian, Y; Wertheimer, SJ; Ahmad, M; Cheung, AW; Firooznia, F; Hamilton, MM; Hayden, S; Li, S; Marcopulos, N; McDermott, L; Tan, J; Yun, W; Guo, L; Pamidimukkala, A; Chen, Y; Huang, KS; Ramsey, GB; Whittard, T; Conde-Knape, K; Taub, R; Rondinone, CM; Tilley, J; Bolin, D Discovery of orally active carboxylic acid derivatives of 2-phenyl-5-trifluoromethyloxazole-4-carboxamide as potent diacylglycerol acyltransferase-1 inhibitors for the potential treatment of obesity and diabetes. J Med Chem54:2433-46 (2011) [PubMed] Article