

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341785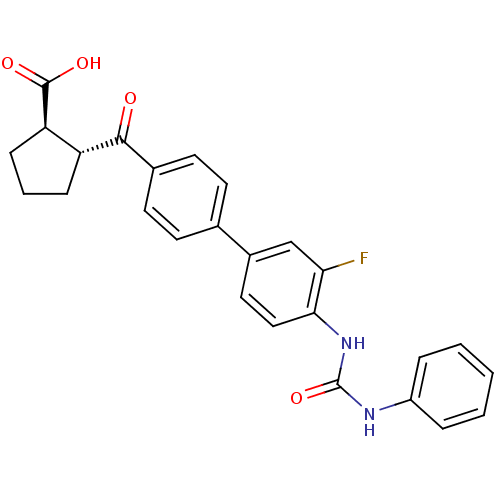 ((1R,2R)-2-(3'-fluoro-4'-(3-phenylureido)biphenylca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341766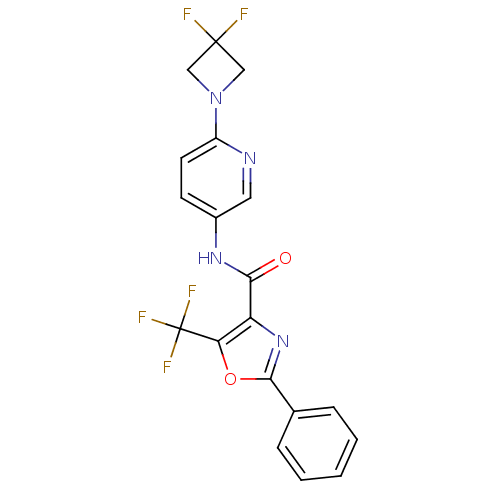 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341768 (4-{5-[(2-Phenyl-5-trifluoromethyloxazole-4-carbony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341778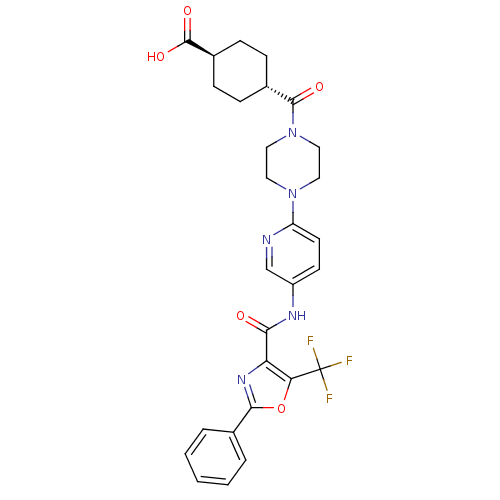 (4-(4-{5-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341760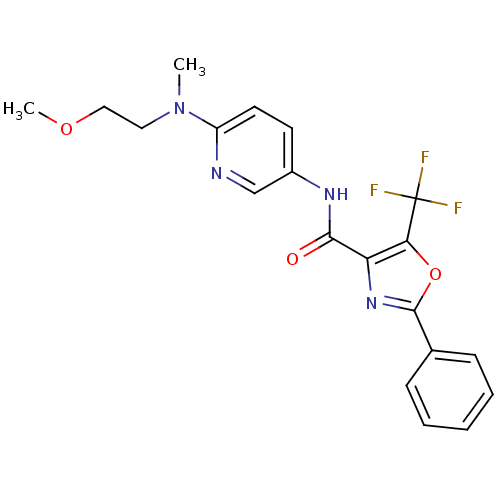 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 using [14C]palmitic acid by TLC analysis | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341760 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341770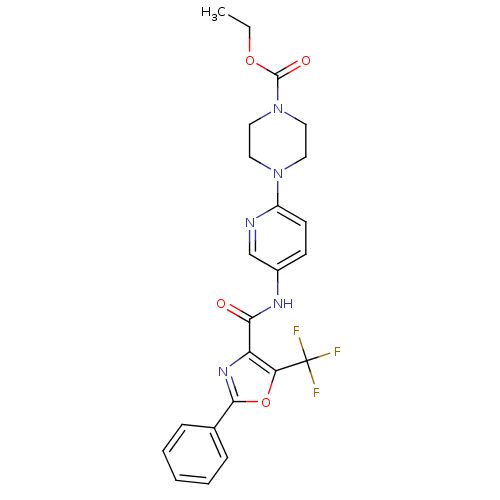 (4-{5-[(2-Phenyl-5-trifluoromethyloxazole-4-carbony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341784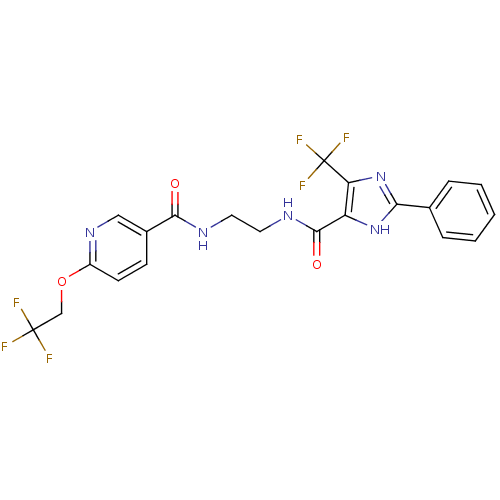 (CHEMBL1766894 | N-(2-(2-phenyl-5-(trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected Sf9 cells using [14C]oleoyl-CoA after 15 mins by TLC analysis | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341763 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341782 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341769 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498901 (CHEMBL3735517) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498904 (CHEMBL3734991) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498903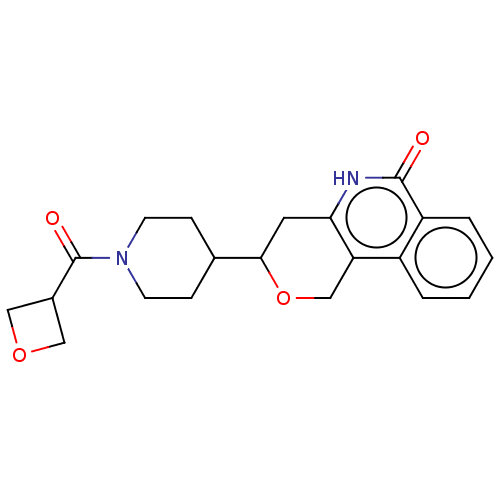 (CHEMBL3735964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498905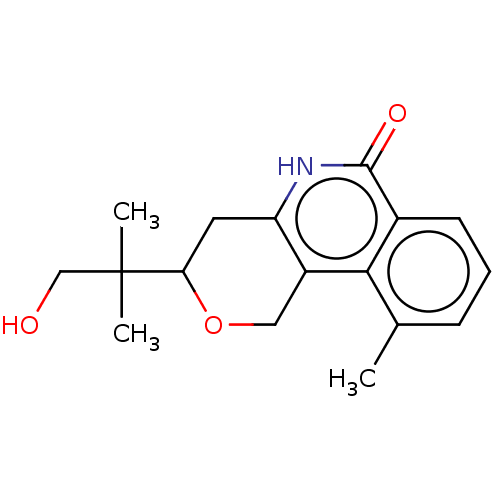 (CHEMBL3736012) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498898 (CHEMBL3736494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498900 (CHEMBL3736145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498897 (CHEMBL3734971) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341776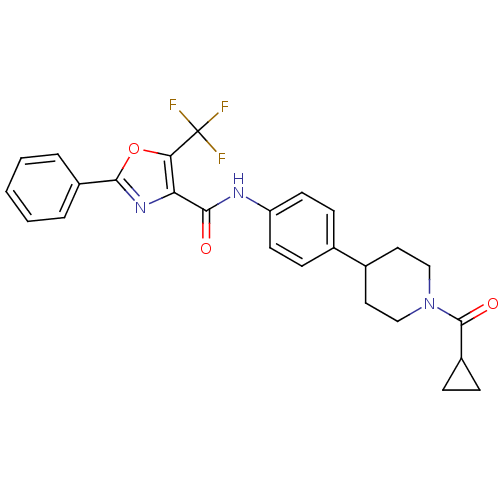 (CHEMBL1766805 | N-(4-(1-(cyclopropanecarbonyl)pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341764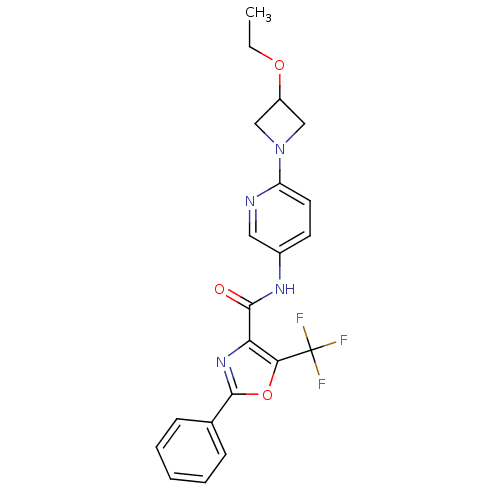 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341771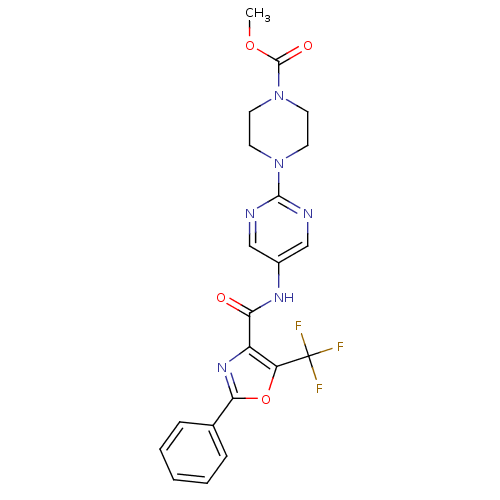 (4-{5-[(2-Phenyl-5-trifluoromethyloxazole-4-carbony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341781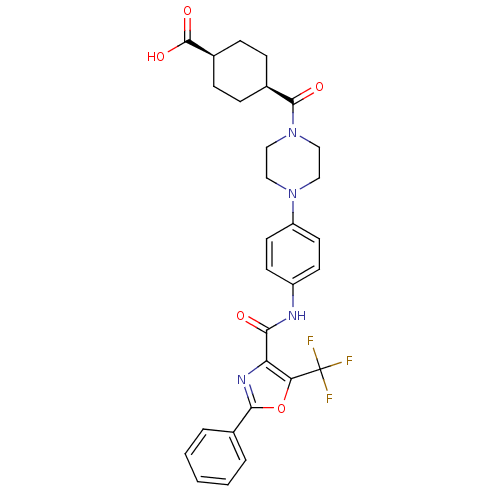 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341783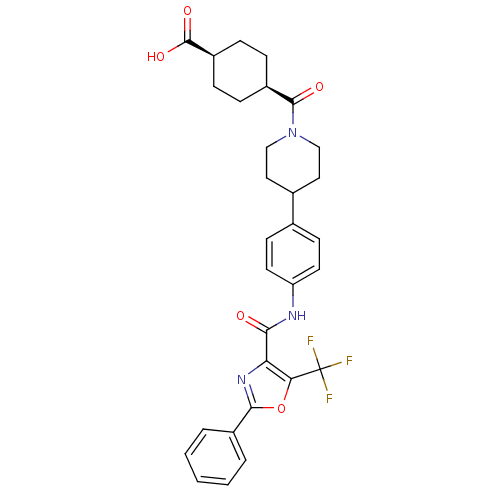 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341777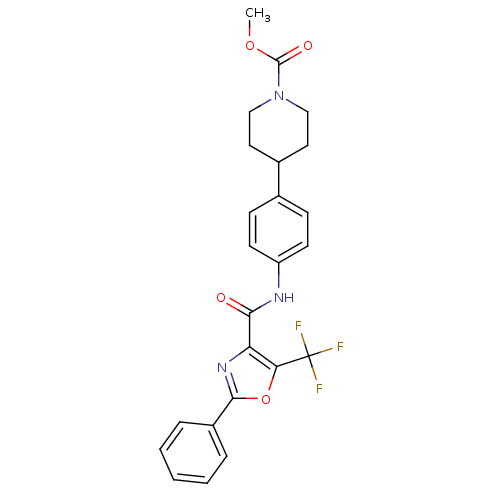 (4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carbony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341772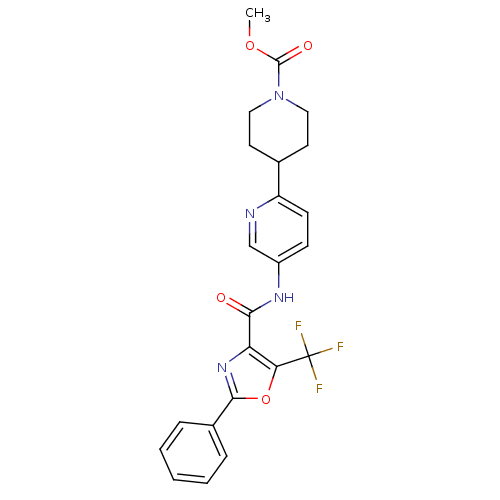 (5-[(2-Phenyl-5-trifluoromethyloxazole-4-carbonyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498899 (CHEMBL3736005) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM27947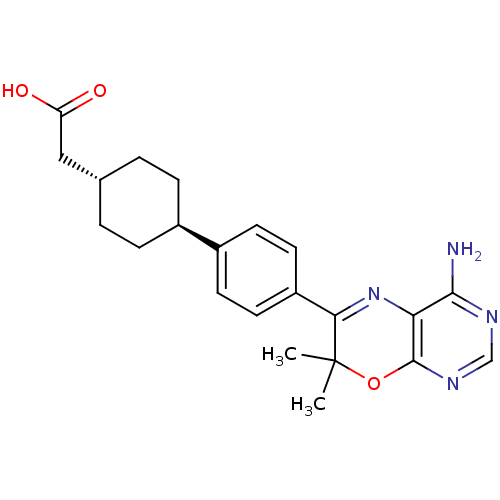 (2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341780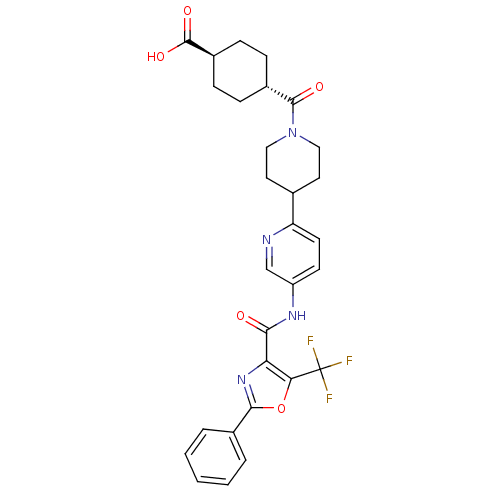 (4-{5-[(2-Phenyl-5-trifluoromethyloxazole-4-carbony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341773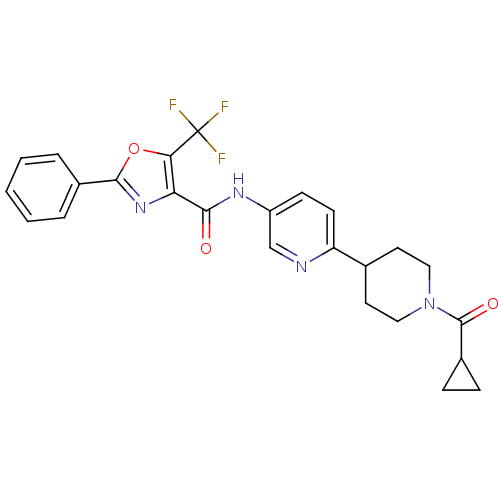 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341775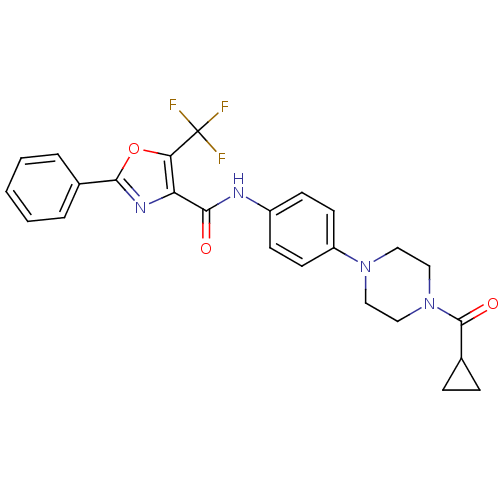 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50321122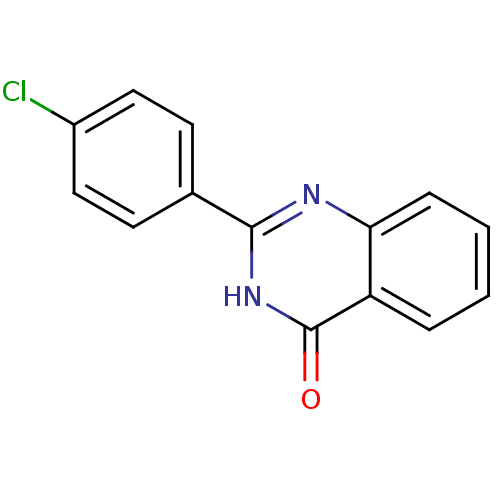 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS1 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341774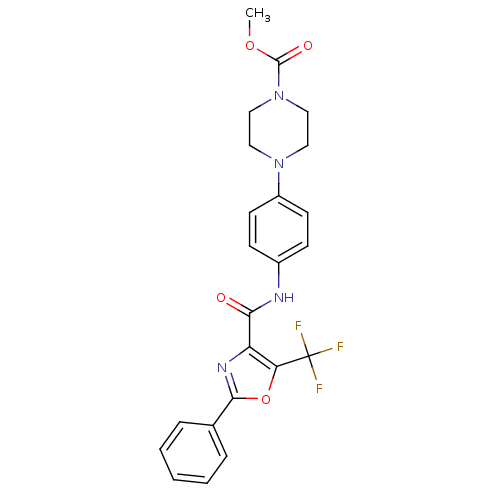 (4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carbony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341767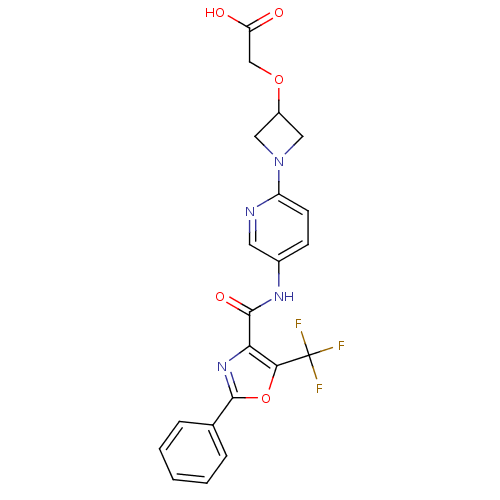 ((1-{5-[(2-Phenyl-5-trifluoromethyloxazole-4-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341765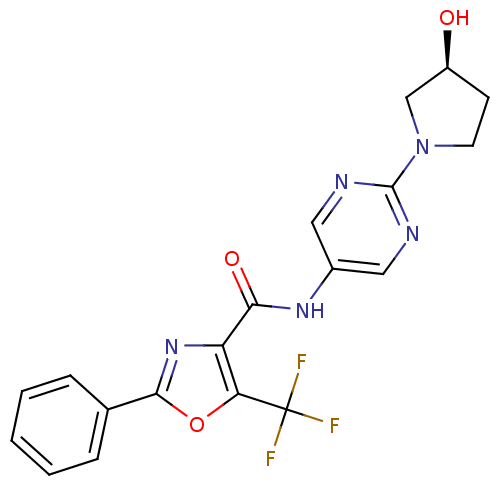 ((S)-2-Phenyl-5-trifluoromethyloxazole-4-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341779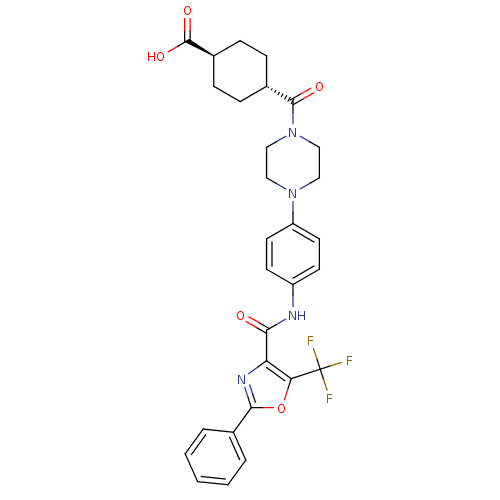 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341762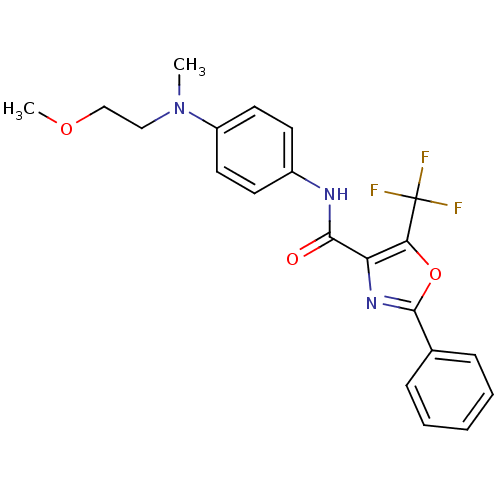 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50498902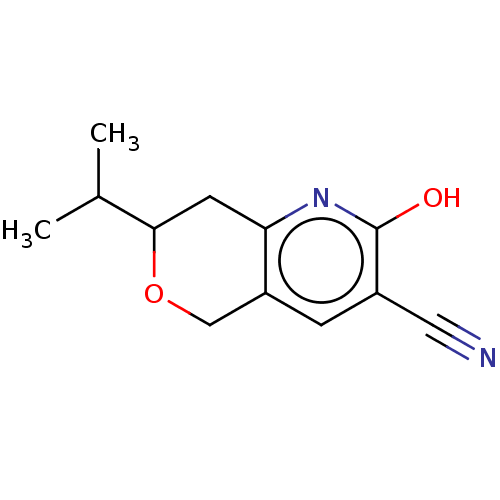 (CHEMBL1527375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS1 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50498902 (CHEMBL1527375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341759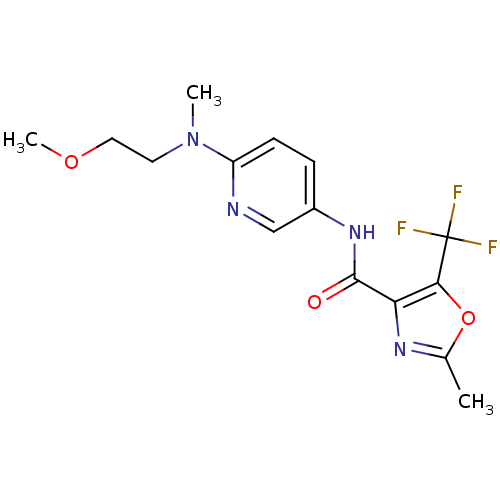 (2-Methyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50012905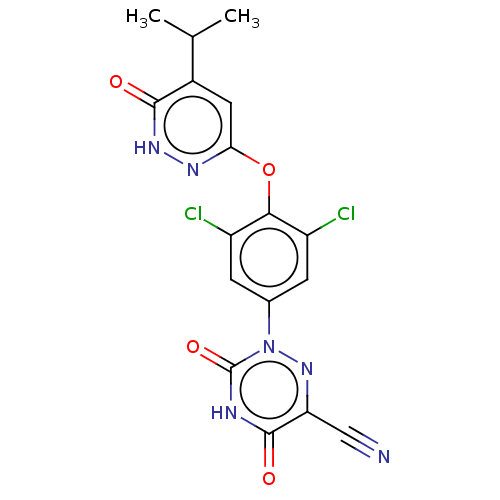 (CHEMBL3261331 | US20240059676, Compound MGL-3196 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 57: 3912-23 (2014) Article DOI: 10.1021/jm4019299 BindingDB Entry DOI: 10.7270/Q23T9JRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341761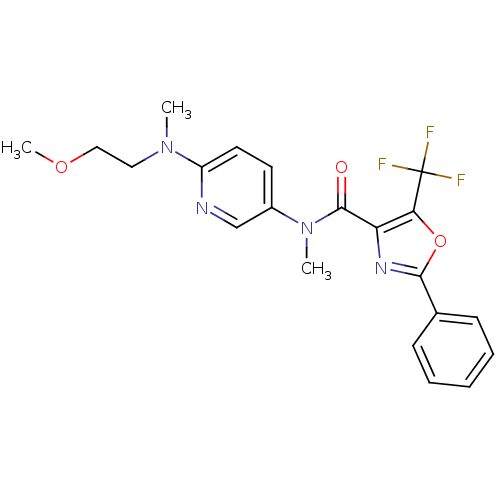 (2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assay | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50341782 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50341782 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50341782 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50012905 (CHEMBL3261331 | US20240059676, Compound MGL-3196 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 57: 3912-23 (2014) Article DOI: 10.1021/jm4019299 BindingDB Entry DOI: 10.7270/Q23T9JRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50012905 (CHEMBL3261331 | US20240059676, Compound MGL-3196 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A5 (unknown origin) | J Med Chem 57: 3912-23 (2014) Article DOI: 10.1021/jm4019299 BindingDB Entry DOI: 10.7270/Q23T9JRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50341782 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50012905 (CHEMBL3261331 | US20240059676, Compound MGL-3196 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 57: 3912-23 (2014) Article DOI: 10.1021/jm4019299 BindingDB Entry DOI: 10.7270/Q23T9JRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50341782 (4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of human ACAT1 expressed in human HepG2 cells after incubated with compound for 15 mins measured after 1 hr using palmitoyl-1-14C-coenzyme... | J Med Chem 54: 2433-46 (2011) Article DOI: 10.1021/jm101580m BindingDB Entry DOI: 10.7270/Q2S182TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 157 total ) | Next | Last >> |