| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Rho-associated protein kinase 1 |
|---|
| Ligand | BDBM14032 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_807416 (CHEMBL1960787) |
|---|
| IC50 | <10±n/a nM |
|---|
| Citation |  Doe, C; Bentley, R; Behm, DJ; Lafferty, R; Stavenger, R; Jung, D; Bamford, M; Panchal, T; Grygielko, E; Wright, LL; Smith, GK; Chen, Z; Webb, C; Khandekar, S; Yi, T; Kirkpatrick, R; Dul, E; Jolivette, L; Marino, JP; Willette, R; Lee, D; Hu, E Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther320:89-98 (2006) [PubMed] Article Doe, C; Bentley, R; Behm, DJ; Lafferty, R; Stavenger, R; Jung, D; Bamford, M; Panchal, T; Grygielko, E; Wright, LL; Smith, GK; Chen, Z; Webb, C; Khandekar, S; Yi, T; Kirkpatrick, R; Dul, E; Jolivette, L; Marino, JP; Willette, R; Lee, D; Hu, E Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther320:89-98 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Rho-associated protein kinase 1 |
|---|
| Name: | Rho-associated protein kinase 1 |
|---|
| Synonyms: | ROCK-I | ROCK1 | ROCK1_HUMAN | Renal carcinoma antigen NY-REN-35 | Rho-associated protein kinase | Rho-associated protein kinase 1 (ROCK1) | Rho-associated, coiled-coil-containing protein kinase 1 | Rho-associated, coiled-coil-containing protein kinase I | Rho-kinase (ROCK I) | Serine/threonine-protein kinase RIO1 | p160 ROCK-1 | p160ROCK |
|---|
| Type: | Serine/threonine-protein kinase |
|---|
| Mol. Mass.: | 158156.77 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q13464 |
|---|
| Residue: | 1354 |
|---|
| Sequence: | MSTGDSFETRFEKMDNLLRDPKSEVNSDCLLDGLDALVYDLDFPALRKNKNIDNFLSRYK
DTINKIRDLRMKAEDYEVVKVIGRGAFGEVQLVRHKSTRKVYAMKLLSKFEMIKRSDSAF
FWEERDIMAFANSPWVVQLFYAFQDDRYLYMVMEYMPGGDLVNLMSNYDVPEKWARFYTA
EVVLALDAIHSMGFIHRDVKPDNMLLDKSGHLKLADFGTCMKMNKEGMVRCDTAVGTPDY
ISPEVLKSQGGDGYYGRECDWWSVGVFLYEMLVGDTPFYADSLVGTYSKIMNHKNSLTFP
DDNDISKEAKNLICAFLTDREVRLGRNGVEEIKRHLFFKNDQWAWETLRDTVAPVVPDLS
SDIDTSNFDDLEEDKGEEETFPIPKAFVGNQLPFVGFTYYSNRRYLSSANPNDNRTSSNA
DKSLQESLQKTIYKLEEQLHNEMQLKDEMEQKCRTSNIKLDKIMKELDEEGNQRRNLEST
VSQIEKEKMLLQHRINEYQRKAEQENEKRRNVENEVSTLKDQLEDLKKVSQNSQLANEKL
SQLQKQLEEANDLLRTESDTAVRLRKSHTEMSKSISQLESLNRELQERNRILENSKSQTD
KDYYQLQAILEAERRDRGHDSEMIGDLQARITSLQEEVKHLKHNLEKVEGERKEAQDMLN
HSEKEKNNLEIDLNYKLKSLQQRLEQEVNEHKVTKARLTDKHQSIEEAKSVAMCEMEKKL
KEEREAREKAENRVVQIEKQCSMLDVDLKQSQQKLEHLTGNKERMEDEVKNLTLQLEQES
NKRLLLQNELKTQAFEADNLKGLEKQMKQEINTLLEAKRLLEFELAQLTKQYRGNEGQMR
ELQDQLEAEQYFSTLYKTQVKELKEEIEEKNRENLKKIQELQNEKETLATQLDLAETKAE
SEQLARGLLEEQYFELTQESKKAASRNRQEITDKDHTVSRLEEANSMLTKDIEILRRENE
ELTEKMKKAEEEYKLEKEEEISNLKAAFEKNINTERTLKTQAVNKLAEIMNRKDFKIDRK
KANTQDLRKKEKENRKLQLELNQEREKFNQMVVKHQKELNDMQAQLVEECAHRNELQMQL
ASKESDIEQLRAKLLDLSDSTSVASFPSADETDGNLPESRIEGWLSVPNRGNIKRYGWKK
QYVVVSSKKILFYNDEQDKEQSNPSMVLDIDKLFHVRPVTQGDVYRAETEEIPKIFQILY
ANEGECRKDVEMEPVQQAEKTNFQNHKGHEFIPTLYHFPANCDACAKPLWHVFKPPPALE
CRRCHVKCHRDHLDKKEDLICPCKVSYDVTSARDMLLLACSQDEQKKWVTHLVKKIPKNP
PSGFVRASPRTLSTRSTANQSFRKVVKNTSGKTS
|
|
|
|---|
| BDBM14032 |
|---|
| n/a |
|---|
| Name | BDBM14032 |
|---|
| Synonyms: | 4-(1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-oxadiazol-3-amine | 4-{1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl}-1,2,5-oxadiazol-3-amine | Aminofurazanyl-azabenzimidazole 1 | CHEMBL189657 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H10N6O |
|---|
| Mol. Mass. | 230.226 |
|---|
| SMILES | CCn1c(nc2cnccc12)-c1nonc1N |
|---|
| Structure | 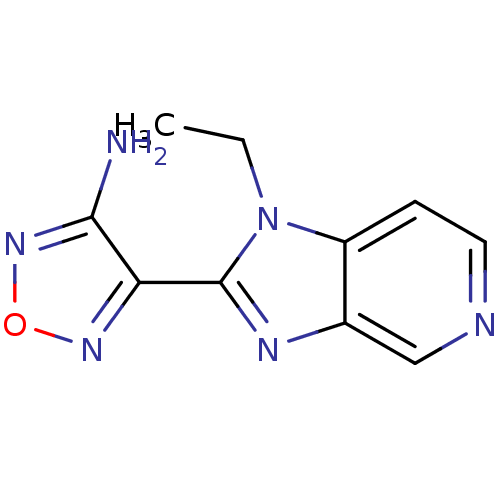
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Doe, C; Bentley, R; Behm, DJ; Lafferty, R; Stavenger, R; Jung, D; Bamford, M; Panchal, T; Grygielko, E; Wright, LL; Smith, GK; Chen, Z; Webb, C; Khandekar, S; Yi, T; Kirkpatrick, R; Dul, E; Jolivette, L; Marino, JP; Willette, R; Lee, D; Hu, E Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther320:89-98 (2006) [PubMed] Article
Doe, C; Bentley, R; Behm, DJ; Lafferty, R; Stavenger, R; Jung, D; Bamford, M; Panchal, T; Grygielko, E; Wright, LL; Smith, GK; Chen, Z; Webb, C; Khandekar, S; Yi, T; Kirkpatrick, R; Dul, E; Jolivette, L; Marino, JP; Willette, R; Lee, D; Hu, E Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther320:89-98 (2006) [PubMed] Article