

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50451322 (CHEMBL4210026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521182 (CHEMBL4456312 | US11260049, Ex. 123) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521192 (CHEMBL4547537 | US11260049, Ex. 121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521183 (CHEMBL4461475 | US11260049, Ex. 125) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435764 (CHEMBL2392692) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451337 (CHEMBL4204601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451338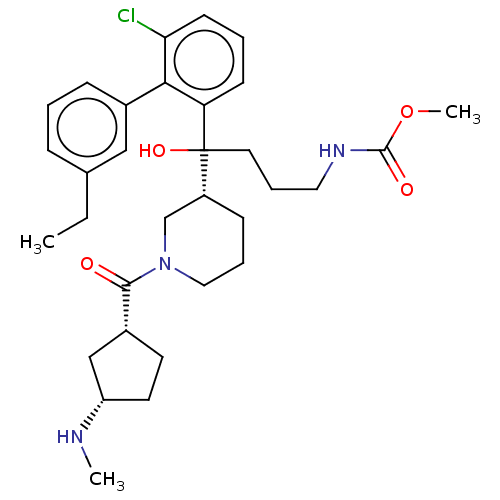 (CHEMBL4207673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451321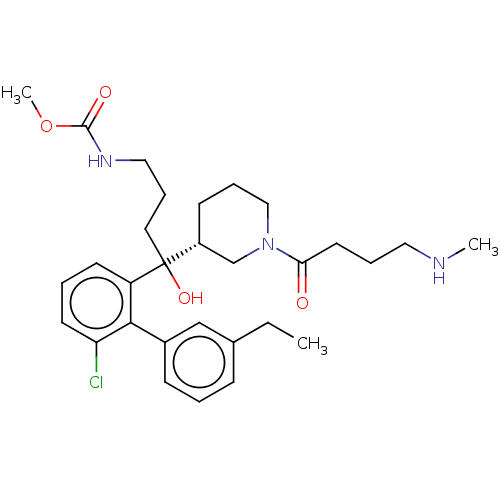 (CHEMBL4211388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14043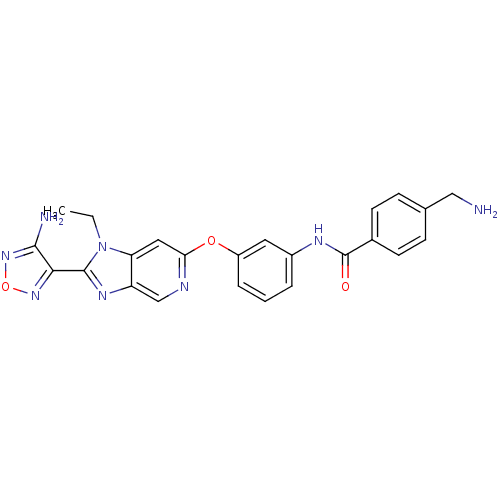 (Aminofurazanyl-azabenzimidazole 6j | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435756 (CHEMBL2392695) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Rattus norvegicus) | BDBM50521183 (CHEMBL4461475 | US11260049, Ex. 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 m... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435758 (CHEMBL2392693) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435755 (CHEMBL2392696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435753 (CHEMBL2392698) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435765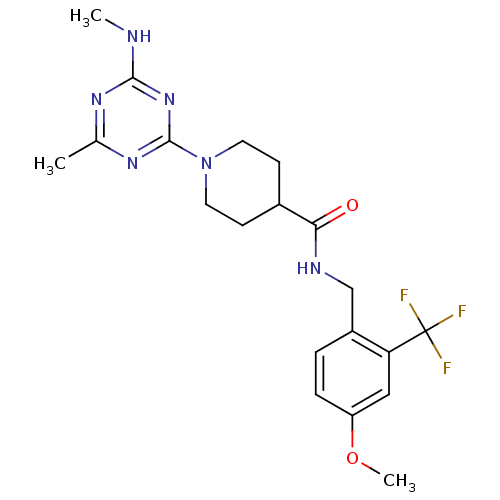 (CHEMBL2392691) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435757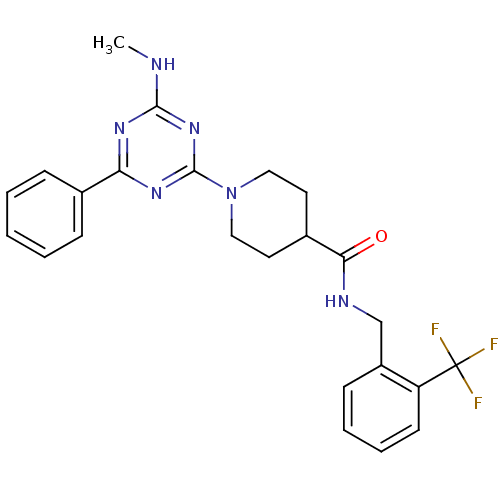 (CHEMBL2392694) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435754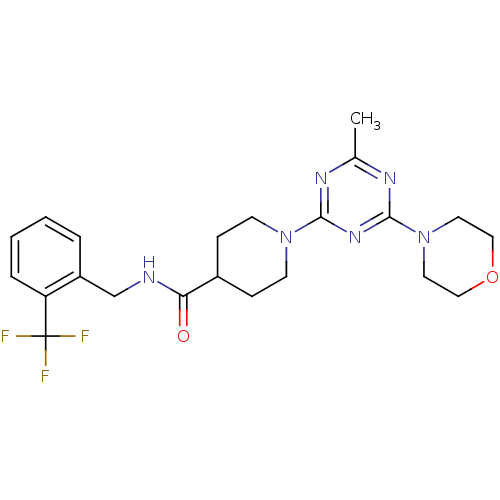 (CHEMBL2392697) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435753 (CHEMBL2392698) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435745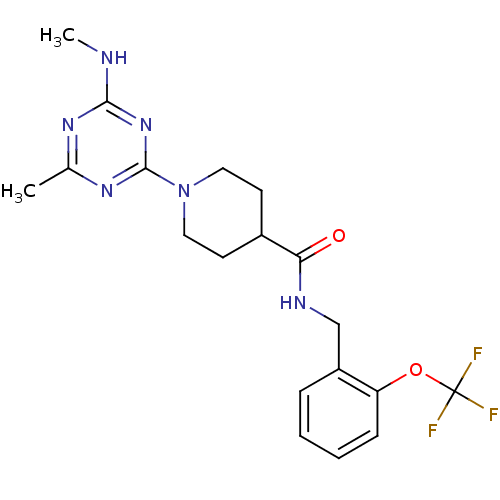 (CHEMBL2392706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451325 (CHEMBL4202424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14046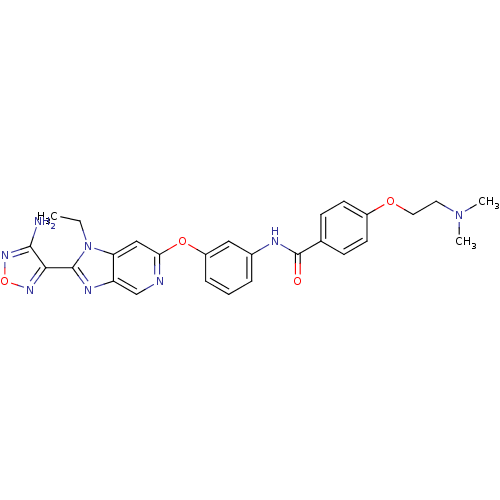 (Aminofurazanyl-azabenzimidazole 6m | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14045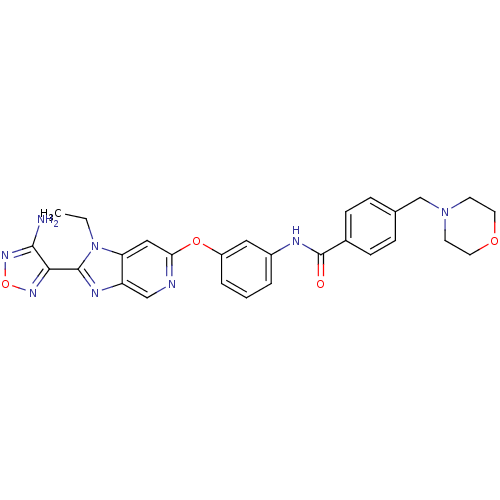 (Aminofurazanyl-azabenzimidazole 6l | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14044 (Aminofurazanyl-azabenzimidazole 6k | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14042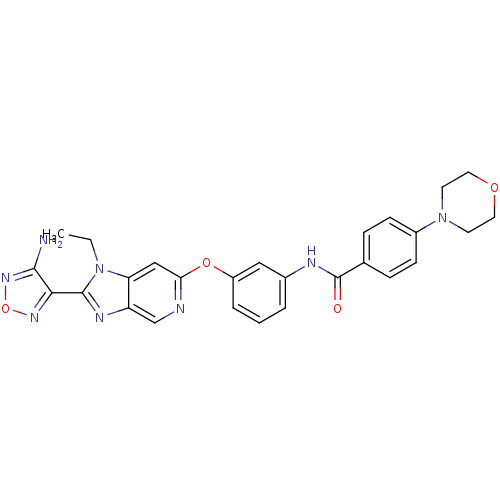 (Aminofurazanyl-azabenzimidazole 6i | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451314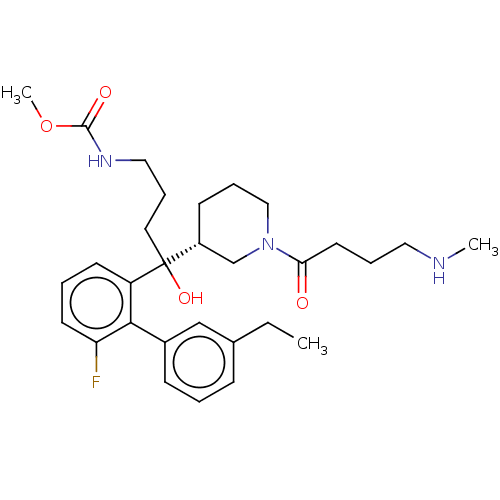 (CHEMBL4207648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50554960 (CHEMBL4749555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV4 expressed in BHK or HEK MSR2 cells assessed as inhibition of TRPV4 agonist GSK634775 (EC80)-induced response incub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01303 BindingDB Entry DOI: 10.7270/Q2XW4PFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451334 (CHEMBL4213138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451318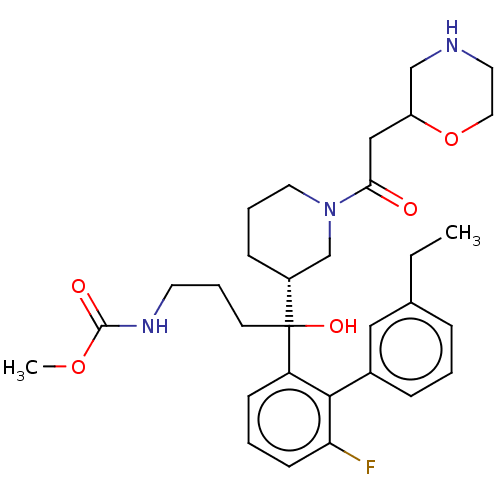 (CHEMBL4218098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451328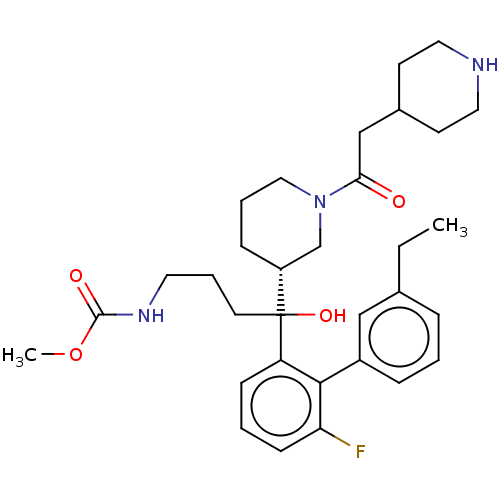 (CHEMBL4211697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14047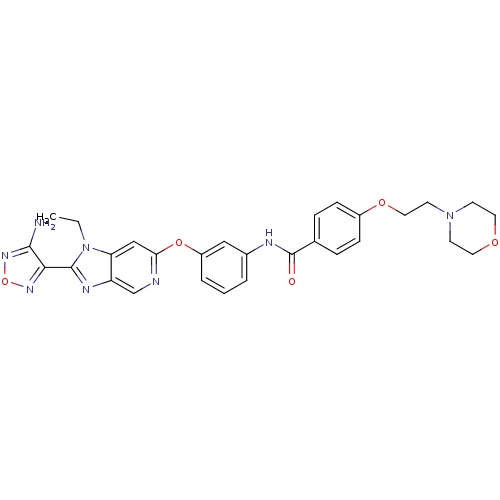 (Aminofurazanyl-azabenzimidazole 6n | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal his-tagged ROCK1 (3-543) expressed in baculovirus infected Sf9 cells using Biotin-Ahx-AKRRLSSLRA-CONH2 sub... | J Pharmacol Exp Ther 320: 89-98 (2006) Article DOI: 10.1124/jpet.106.110635 BindingDB Entry DOI: 10.7270/Q2DV1KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14035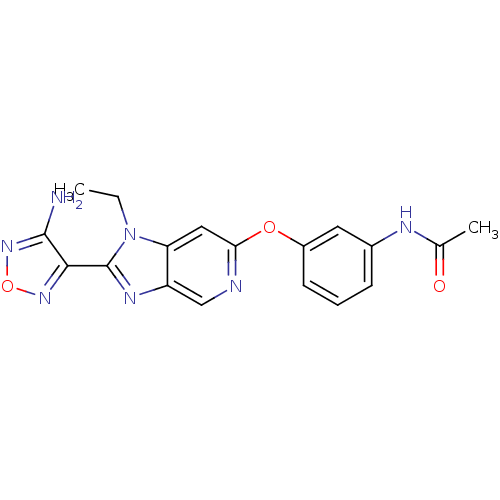 (Aminofurazanyl-azabenzimidazole 6c | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14047 (Aminofurazanyl-azabenzimidazole 6n | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451322 (CHEMBL4210026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of renin in human plasma using angiotensinogen as substrate measured for 90 mins by [125I]-angiotensin based radioimmunoassay | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50502640 (CHEMBL4470585) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 | J Med Chem 62: 9270-9280 (2019) Article DOI: 10.1021/acs.jmedchem.9b01247 BindingDB Entry DOI: 10.7270/Q2N30173 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521195 (CHEMBL4586959 | US11260049, Ex. 84) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521185 (CHEMBL4439448 | US11260049, Ex. 83) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435765 (CHEMBL2392691) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451319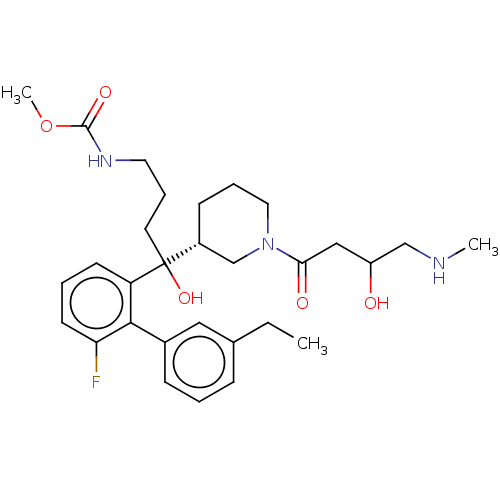 (CHEMBL4212859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25494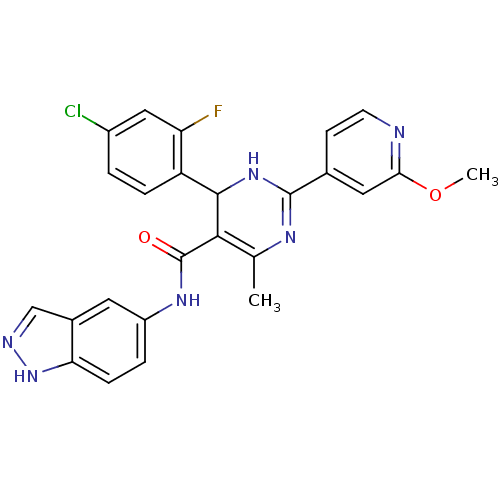 (4-(4-chloro-2-fluorophenyl)-N-(1H-indazol-5-yl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451320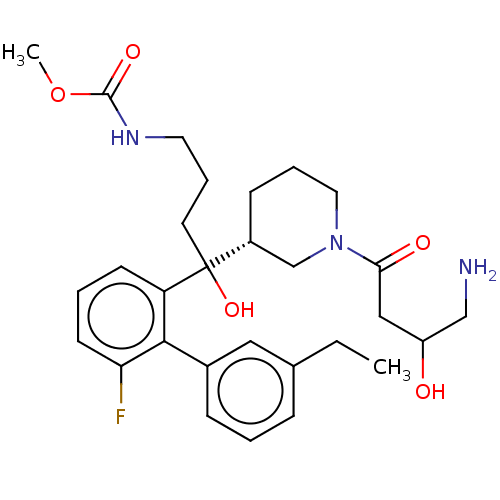 (CHEMBL4216413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451329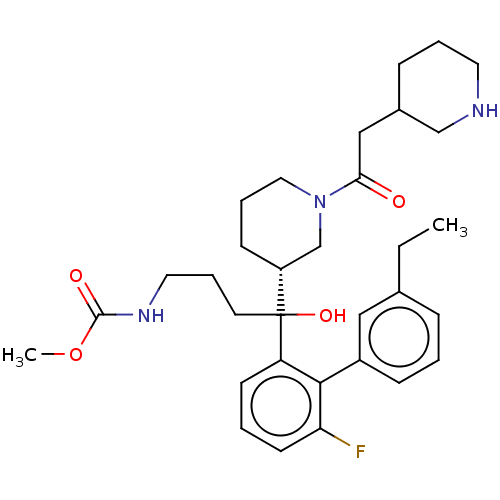 (CHEMBL4212429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451331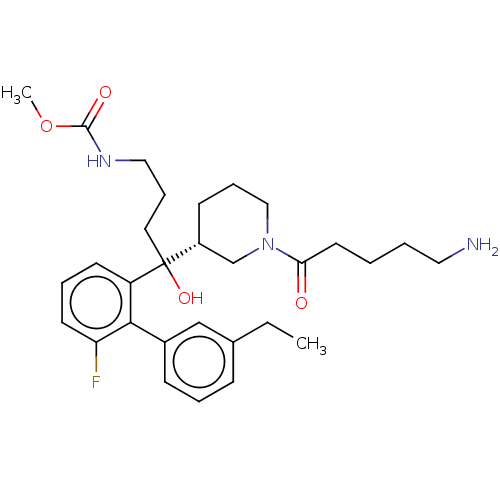 (CHEMBL4217145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50435764 (CHEMBL2392692) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14041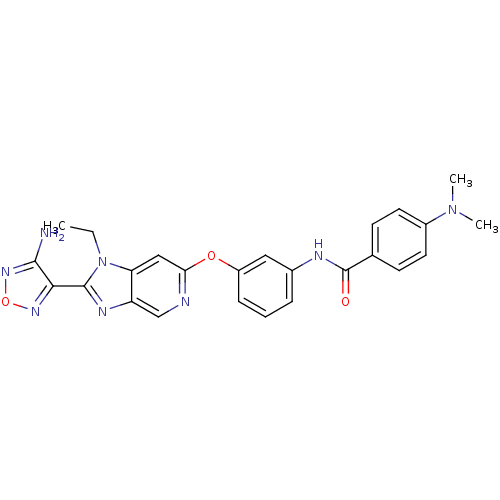 (Aminofurazanyl-azabenzimidazole 6h | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50451317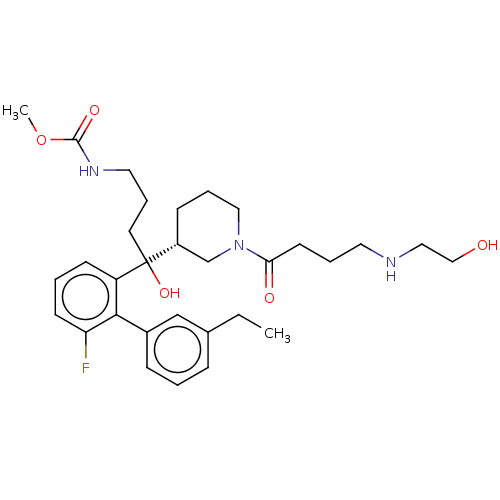 (CHEMBL4202961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of trypsin-activated recombinant human Fc-tagged renin expressed in BacMam virus infected HEK-F cells using Arg-Glu-Lys(5-Fam)-Ile-His-Pro... | Bioorg Med Chem Lett 27: 4838-4843 (2017) Article DOI: 10.1016/j.bmcl.2017.09.046 BindingDB Entry DOI: 10.7270/Q2Z60RN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435764 (CHEMBL2392692) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435745 (CHEMBL2392706) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435754 (CHEMBL2392697) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50245305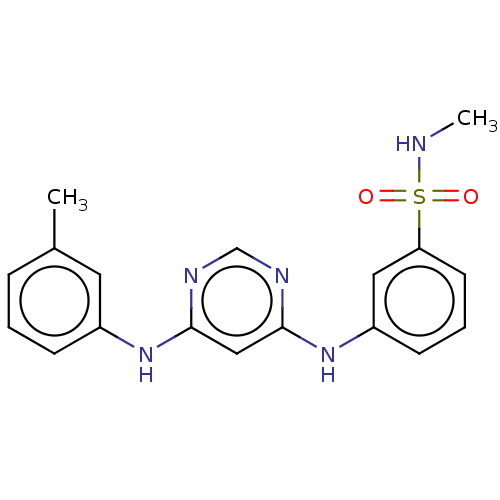 (CHEMBL4097027) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | J Med Chem 61: 3076-3088 (2018) Article DOI: 10.1021/acs.jmedchem.8b00125 BindingDB Entry DOI: 10.7270/Q2Z3221C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25495 (4-(4-chloro-2-fluorophenyl)-N-(1H-indazol-5-yl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 375 total ) | Next | Last >> |