

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040857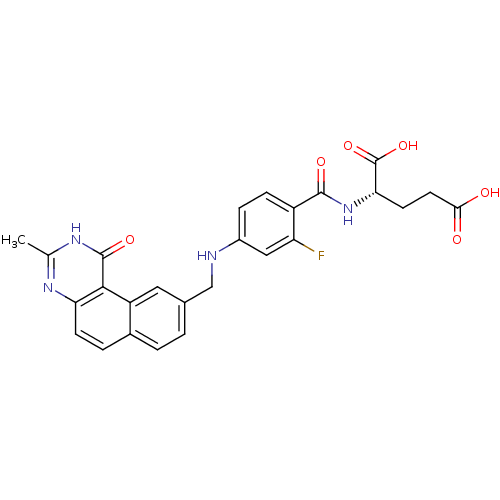 ((S)-2-{2-Fluoro-4-[(3-methyl-1-oxo-1,2-dihydro-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040861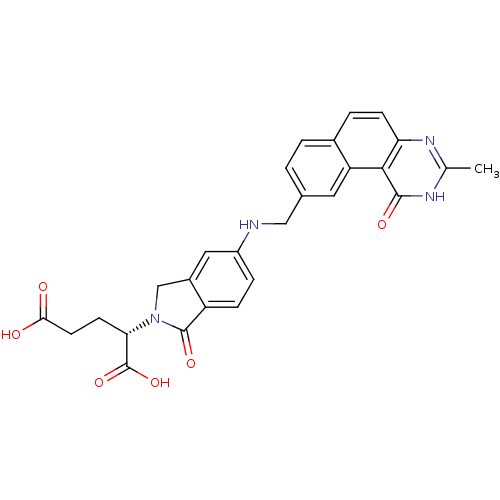 ((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040865 ((S)-2-{4-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of AURA | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040860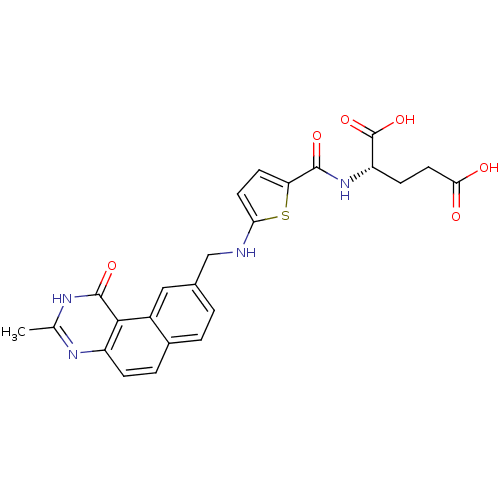 ((S)-2-({5-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040863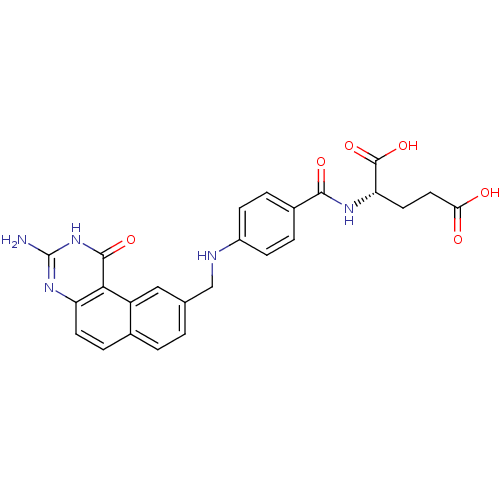 ((S)-2-{4-[(3-Amino-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040859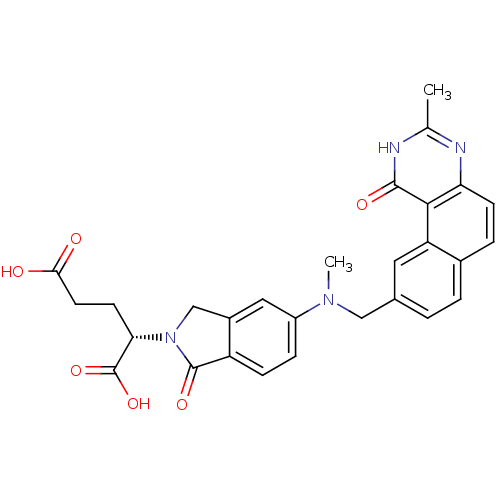 ((S)-2-{5-[Methyl-(3-methyl-1-oxo-1,2-dihydro-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042378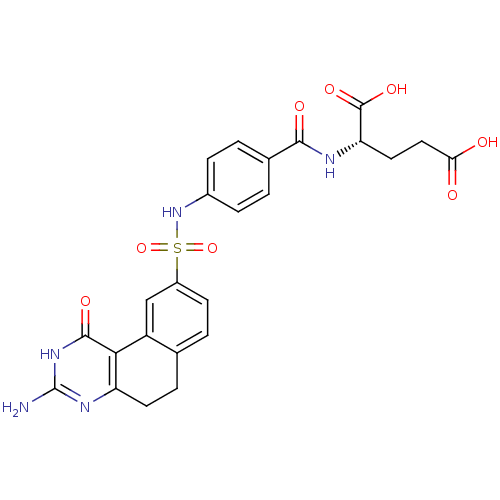 (2-[4-(3-Amino-1-oxo-1,2,5,6-tetrahydro-benzo[f]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040862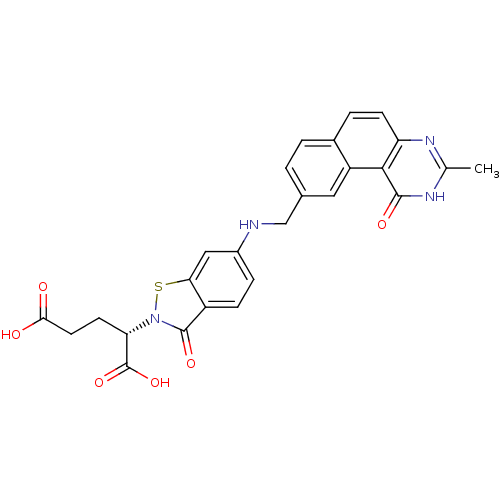 ((S)-2-{6-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of AURC | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50230828 (5-(4-fluorophenyl)-2-ureidothiophene-3-carboxamide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040858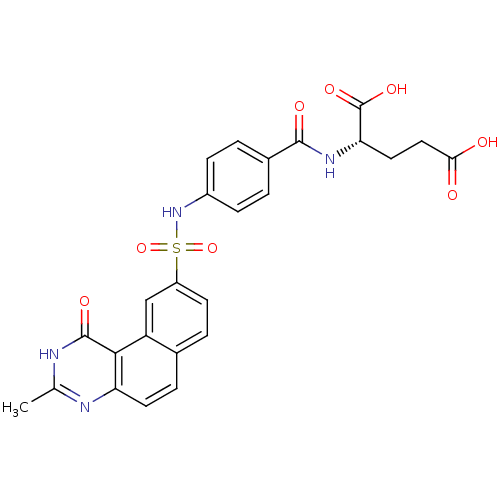 ((S)-2-[4-(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040858 ((S)-2-[4-(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042375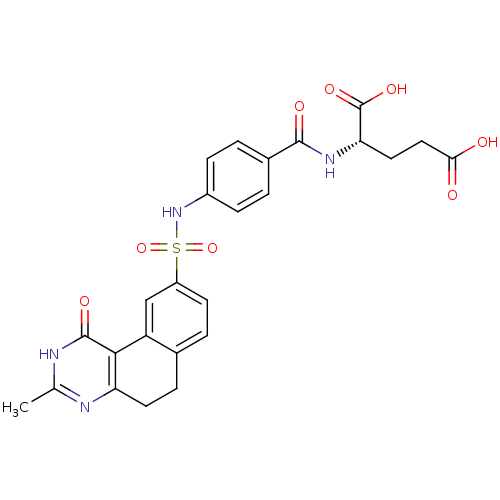 (2-[4-(3-Methyl-1-oxo-1,2,5,6-tetrahydro-benzo[f]qu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of AURB | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50230985 (CHEMBL306705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase (TS) isolated from an Escherichia coli harboring a plasmid with thy A gene cloned from SV40 transfo... | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040864 ((S)-2-{4-[Methyl-(3-methyl-1-oxo-1,2-dihydro-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human FLT3 | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042382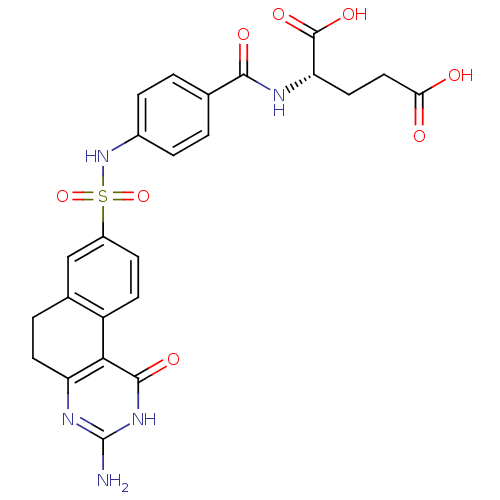 (2-[4-(3-Amino-1-oxo-1,2,5,6-tetrahydro-benzo[f]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042380 (2-[4-(3-Amino-8-bromo-1-oxo-1,2,5,6-tetrahydro-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50231342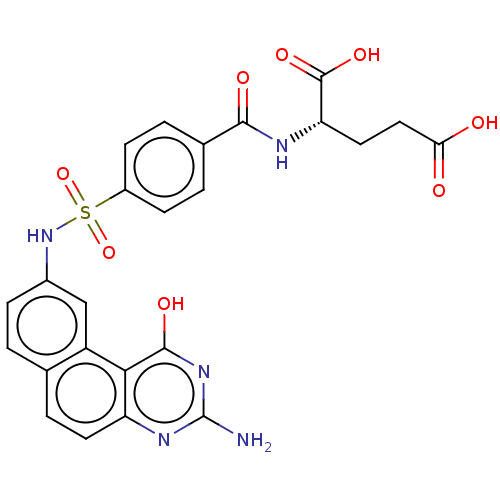 (CHEMBL118750) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase (TS) isolated from an Escherichia coli harboring a plasmid with thy A gene cloned from SV40 transfo... | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042376 (2-{[4-(3-Amino-1-oxo-1,2,5,6-tetrahydro-benzo[f]qu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042381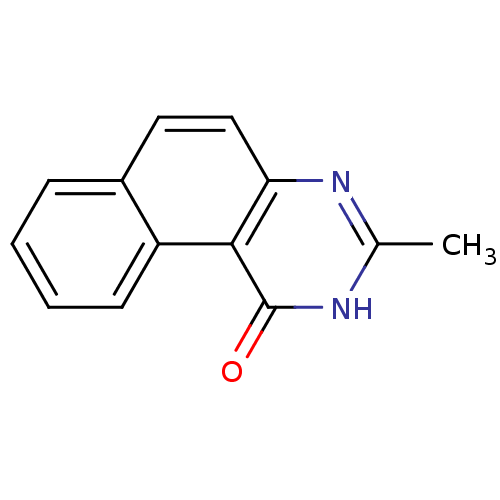 (3-Methyl-2H-benzo[f]quinazolin-1-one | CHEMBL41889...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042383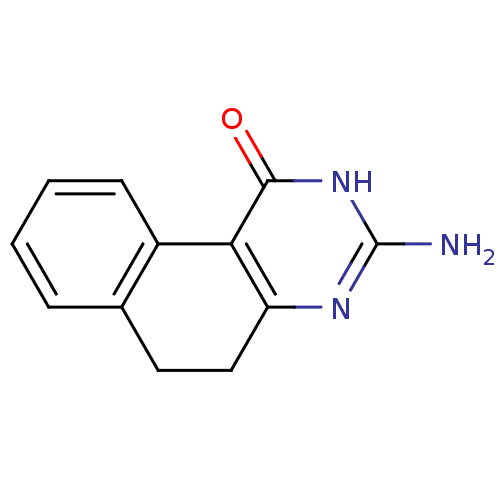 (3-Amino-5,6-dihydro-2H-benzo[f]quinazolin-1-one | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042373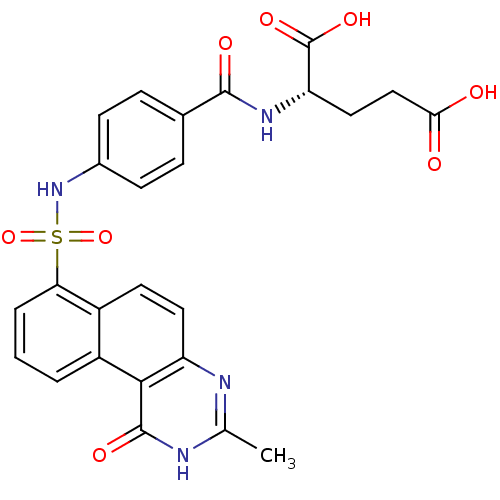 (2-[4-(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quinazoli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042377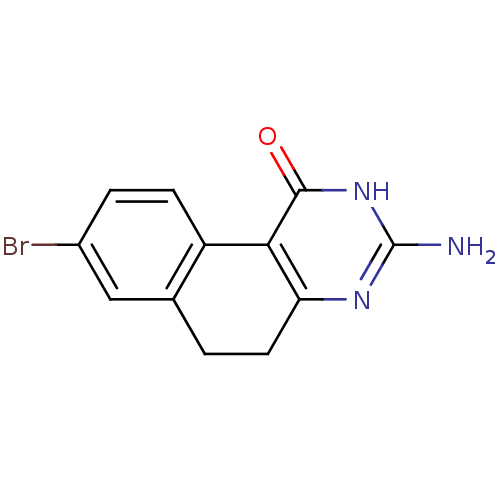 (3-Amino-8-bromo-5,6-dihydro-2H-benzo[f]quinazolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50231341 (CHEMBL116508) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase (TS) isolated from an Escherichia coli harboring a plasmid with thy A gene cloned from SV40 transfo... | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042374 (2-{Methyl-[4-(3-methyl-1-oxo-1,2,5,6-tetrahydro-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042379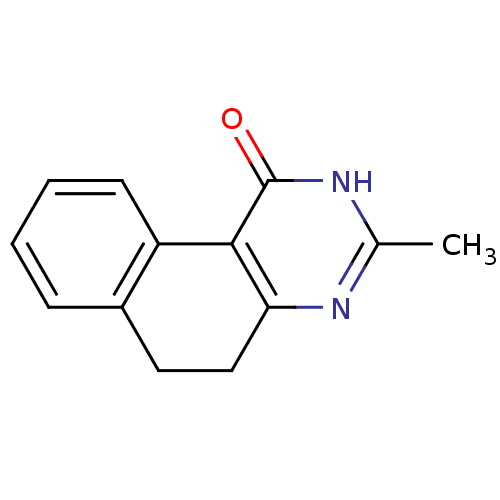 (3-Methyl-5,6-dihydro-2H-benzo[f]quinazolin-1-one |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14046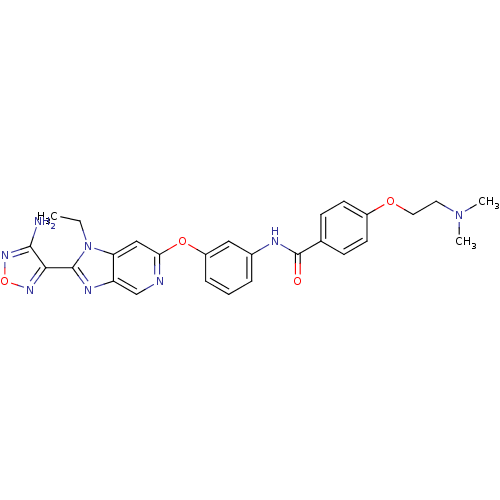 (Aminofurazanyl-azabenzimidazole 6m | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14045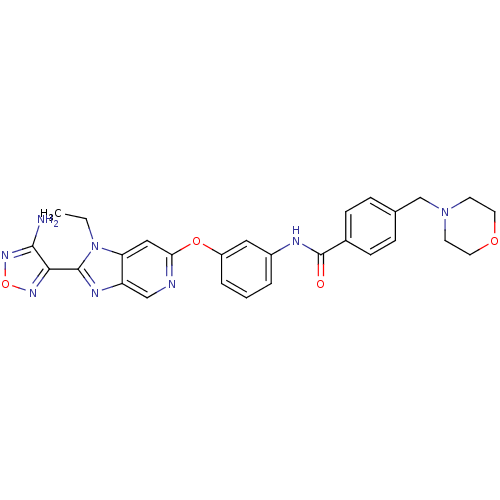 (Aminofurazanyl-azabenzimidazole 6l | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14044 (Aminofurazanyl-azabenzimidazole 6k | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14043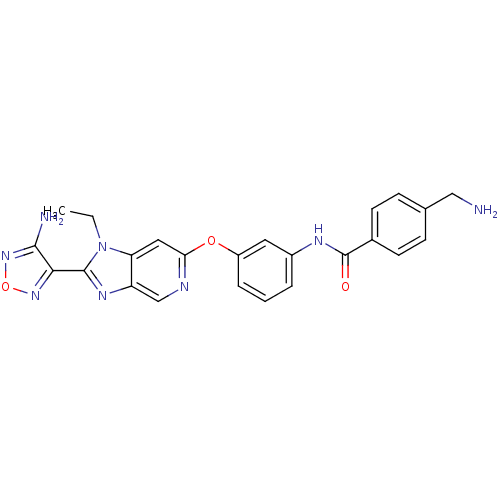 (Aminofurazanyl-azabenzimidazole 6j | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14042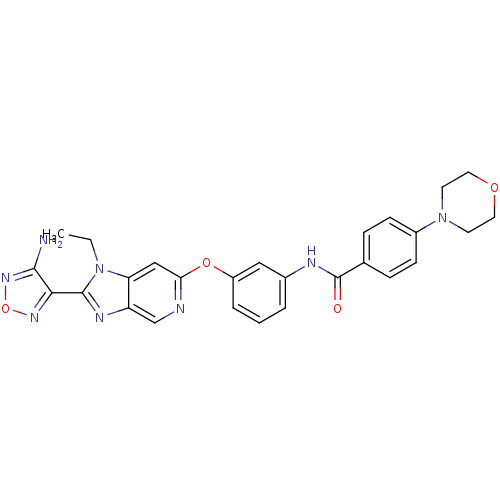 (Aminofurazanyl-azabenzimidazole 6i | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14047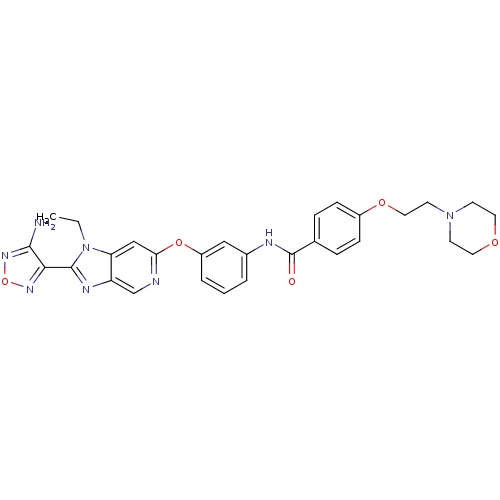 (Aminofurazanyl-azabenzimidazole 6n | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal his-tagged ROCK1 (3-543) expressed in baculovirus infected Sf9 cells using Biotin-Ahx-AKRRLSSLRA-CONH2 sub... | J Pharmacol Exp Ther 320: 89-98 (2006) Article DOI: 10.1124/jpet.106.110635 BindingDB Entry DOI: 10.7270/Q2DV1KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14047 (Aminofurazanyl-azabenzimidazole 6n | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14035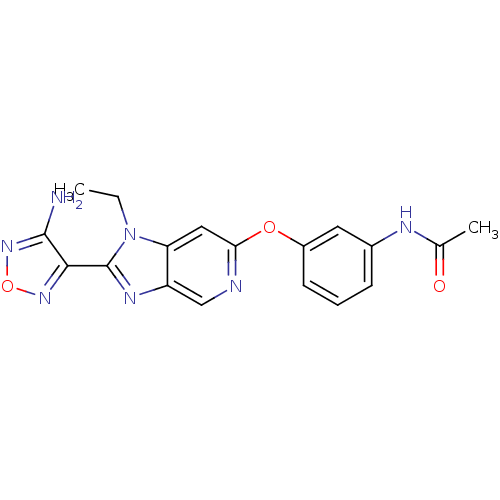 (Aminofurazanyl-azabenzimidazole 6c | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14041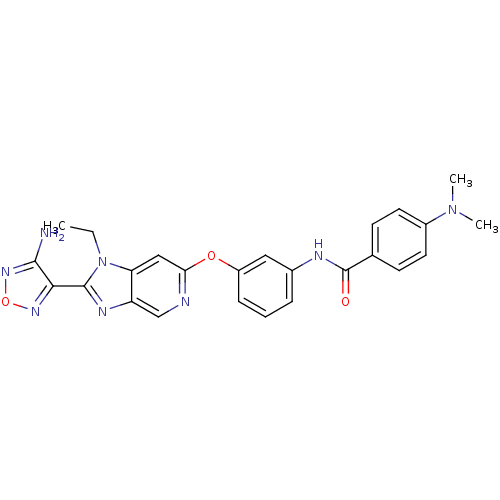 (Aminofurazanyl-azabenzimidazole 6h | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25494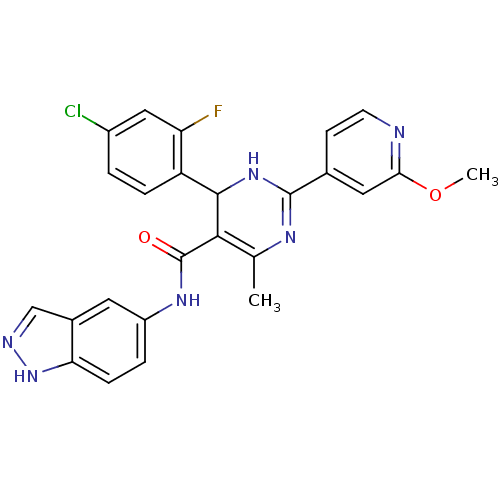 (4-(4-chloro-2-fluorophenyl)-N-(1H-indazol-5-yl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25495 (4-(4-chloro-2-fluorophenyl)-N-(1H-indazol-5-yl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM14047 (Aminofurazanyl-azabenzimidazole 6n | N-(3-{[2-(4-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human ROCK2 | J Pharmacol Exp Ther 320: 89-98 (2006) Article DOI: 10.1124/jpet.106.110635 BindingDB Entry DOI: 10.7270/Q2DV1KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14040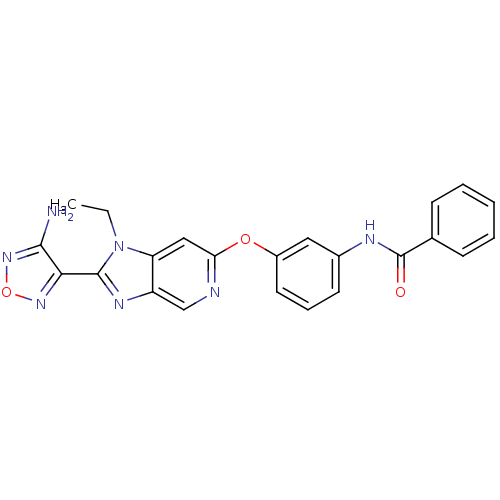 (Aminofurazanyl-azabenzimidazole 6g | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25493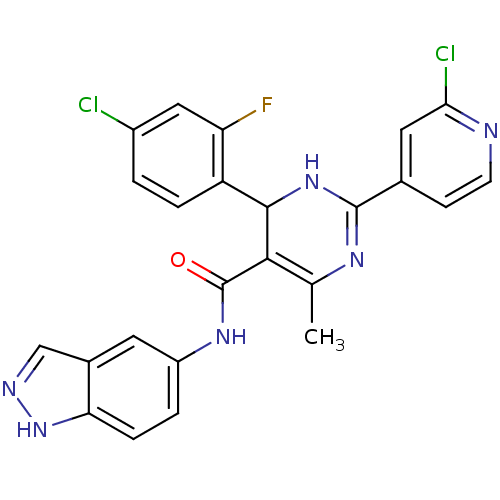 (4-(4-chloro-2-fluorophenyl)-2-(2-chloropyridin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14033 (4-{1-ethyl-6-methoxy-1H-imidazo[4,5-c]pyridin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25492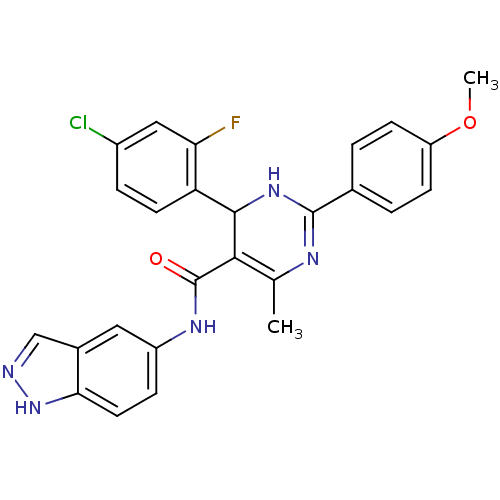 (4-(4-chloro-2-fluorophenyl)-N-(1H-indazol-5-yl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14050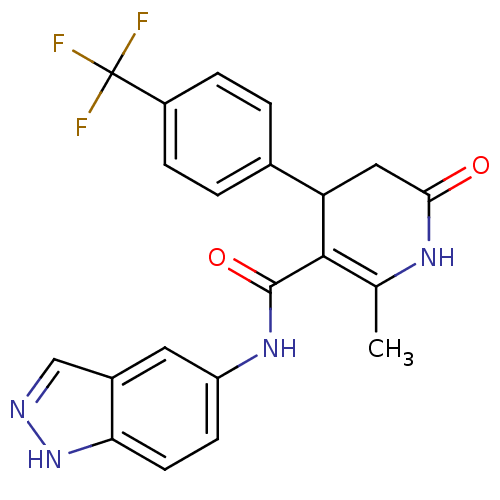 (N-(1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50365218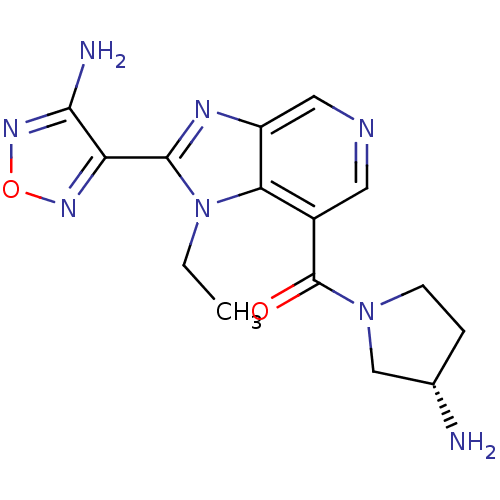 (CHEMBL1956071 | GSK screening, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal his-tagged ROCK1 (3-543) expressed in baculovirus infected Sf9 cells using Biotin-Ahx-AKRRLSSLRA-CONH2 sub... | J Pharmacol Exp Ther 320: 89-98 (2006) Article DOI: 10.1124/jpet.106.110635 BindingDB Entry DOI: 10.7270/Q2DV1KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25498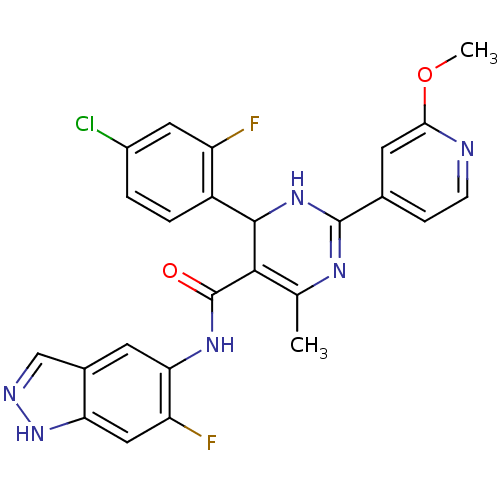 (4-(4-chloro-2-fluorophenyl)-N-(6-fluoro-1H-indazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50365218 (CHEMBL1956071 | GSK screening, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human ROCK2 | J Pharmacol Exp Ther 320: 89-98 (2006) Article DOI: 10.1124/jpet.106.110635 BindingDB Entry DOI: 10.7270/Q2DV1KBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM25496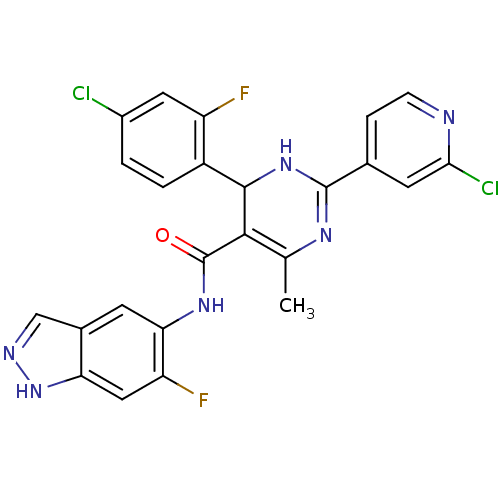 (4-(4-chloro-2-fluorophenyl)-2-(2-chloropyridin-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... | J Med Chem 51: 6631-4 (2008) Article DOI: 10.1021/jm8005096 BindingDB Entry DOI: 10.7270/Q2F47MFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 248 total ) | Next | Last >> |