| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aminopeptidase B |
|---|
| Ligand | BDBM50078122 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_35337 |
|---|
| Ki | >0.000±n/a nM |
|---|
| Comments | >0.0000100 01/04/22 |
|---|
| Citation |  Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed] Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aminopeptidase B |
|---|
| Name: | Aminopeptidase B |
|---|
| Synonyms: | AMPB_MOUSE | AP-B | Arginine aminopeptidase | Arginyl aminopeptidase | Cytosol aminopeptidase IV | Rnpep |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 72401.87 |
|---|
| Organism: | Mus musculus |
|---|
| Description: | ChEMBL_35337 |
|---|
| Residue: | 650 |
|---|
| Sequence: | MESGGPGNYSAAARRPLHSAQAVDVASASSFRAFEILHLHLDLRAEFGPPGPGPGSRGLS
GTATLELRCLLPEGASELRLDSHSCLEVTAATLRRGQPGDQQAPAEPVPFHTQPFSHYGQ
ALCVAFRQPCGAADRFELELTYRVGEGPGVCWLAPEQTAGKKKPFVYTQGQAVLNRAFFP
CFDTPAVKCTYSALIEVPDGFTAVMSADTWEKRGPNKFFFQMSHPIPSYLIALAIGDLAS
AEVGPRSRVWAEPCLIEAAKEEYSGVIEEFLATGEKLFGPYVWGRYDLLFMPPSFPFGGM
ENPCLTFVTPCLLAGDRSLADVIIHEISHSWFGNLVTNANWGEFWLNEGFTMYAQRRIST
ILFGAAYTCLEAATGRALLRQHMNVSGEENPLNKLRVKIEPGVDPDDTYNETPYEKGYCF
VSYLAHLVGDQDQFDKFLKAYVDEFKFQSILAEDFLEFYLEYFPELKKKGVDSIPGFEFD
RWLNTPGWPPYLPDLSPGDSLMKPAEELAELWVTSEPDMQAIEAVAISTWKTYQLVYFLD
KILQKSPLPPGNVKKLGETYPKISNAQNAELRLRWGQIILKNDYQEEFQKVKDFLQSQGK
QKYTLPLYHAMMGGSEMARTLAKDTFAATASQLHSNVVNYVQQILAPKDS
|
|
|
|---|
| BDBM50078122 |
|---|
| n/a |
|---|
| Name | BDBM50078122 |
|---|
| Synonyms: | (R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-ethylcarbamoyl)-3-phenyl-propyl]-hydroxy-phosphinoyl}-2-phenyl-ethyl-ammonium |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H34N2O5P |
|---|
| Mol. Mass. | 509.5532 |
|---|
| SMILES | C[C@@](Cc1ccccc1)(NC(=O)[C@H](Cc1ccccc1)CP(O)(=O)[C@@H]([NH3+])Cc1ccccc1)C(O)=O |
|---|
| Structure | 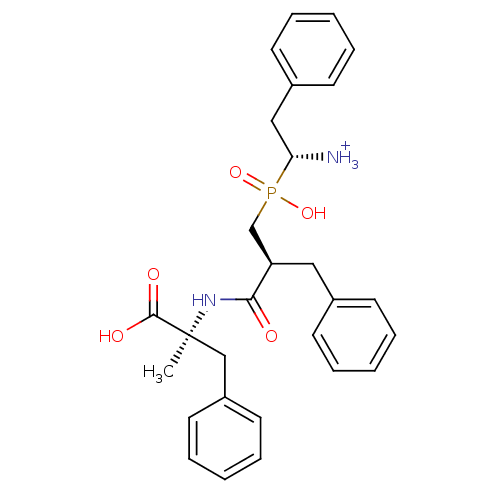
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed]
Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed]