| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aminopeptidase N |
|---|
| Ligand | BDBM50078131 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_35501 (CHEMBL646411) |
|---|
| Ki | 3.2±n/a nM |
|---|
| Citation |  Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed] Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aminopeptidase N |
|---|
| Name: | Aminopeptidase N |
|---|
| Synonyms: | AMPN_PIG | ANPEP | AP-M | AP-N | Alanyl aminopeptidase | Aminopeptidase M | Aminopeptidase N (APN) | CD_antigen=CD13 | Microsomal aminopeptidase | gp130 | pAPN |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 108810.25 |
|---|
| Organism: | Sus scrofa (Pig) |
|---|
| Description: | P15145 |
|---|
| Residue: | 963 |
|---|
| Sequence: | MAKGFYISKALGILGILLGVAAVATIIALSVVYAQEKNKNAEHVPQAPTSPTITTTAAIT
LDQSKPWNRYRLPTTLLPDSYNVTLRPYLTPNADGLYIFKGKSIVRLLCQEPTDVIIIHS
KKLNYTTQGHMVVLRGVGDSQVPEIDRTELVELTEYLVVHLKGSLQPGHMYEMESEFQGE
LADDLAGFYRSEYMEGNVKKVLATTQMQSTDARKSFPCFDEPAMKATFNITLIHPNNLTA
LSNMPPKGSSTPLAEDPNWSVTEFETTPVMSTYLLAYIVSEFQSVNETAQNGVLIRIWAR
PNAIAEGHGMYALNVTGPILNFFANHYNTSYPLPKSDQIALPDFNAGAMENWGLVTYREN
ALLFDPQSSSISNKERVVTVIAHELAHQWFGNLVTLAWWNDLWLNEGFASYVEYLGADHA
EPTWNLKDLIVPGDVYRVMAVDALASSHPLTTPAEEVNTPAQISEMFDSISYSKGASVIR
MLSNFLTEDLFKEGLASYLHAFAYQNTTYLDLWEHLQKAVDAQTSIRLPDTVRAIMDRWT
LQMGFPVITVDTKTGNISQKHFLLDSESNVTRSSAFDYLWIVPISSIKNGVMQDHYWLRD
VSQAQNDLFKTASDDWVLLNVNVTGYFQVNYDEDNWRMIQHQLQTNLSVIPVINRAQVIY
DSFNLATAHMVPVTLALDNTLFLNGEKEYMPWQAALSSLSYFSLMFDRSEVYGPMKKYLR
KQVEPLFQHFETLTKNWTERPENLMDQYSEINAISTACSNGLPQCENLAKTLFDQWMSDP
ENNPIHPNLRSTIYCNAIAQGGQDQWDFAWGQLQQAQLVNEADKLRSALACSNEVWLLNR
YLGYTLNPDLIRKQDATSTINSIASNVIGQPLAWDFVQSNWKKLFQDYGGGSFSFSNLIQ
GVTRRFSSEFELQQLEQFKKNNMDVGFGSGTRALEQALEKTKANIKWVKENKEVVLNWFI
EHS
|
|
|
|---|
| BDBM50078131 |
|---|
| n/a |
|---|
| Name | BDBM50078131 |
|---|
| Synonyms: | 2-{3-[(1-Amino-3-methyl-butyl)-hydroxy-phosphinoyl]-2-benzyl-propionylamino}-3-phenyl-propionic acid | CHEMBL290357 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H33N2O5P |
|---|
| Mol. Mass. | 460.503 |
|---|
| SMILES | CC(C)CC(=N)P(O)(O)CC(Cc1ccccc1)C(=O)NC(Cc1ccccc1)C(O)=O |
|---|
| Structure | 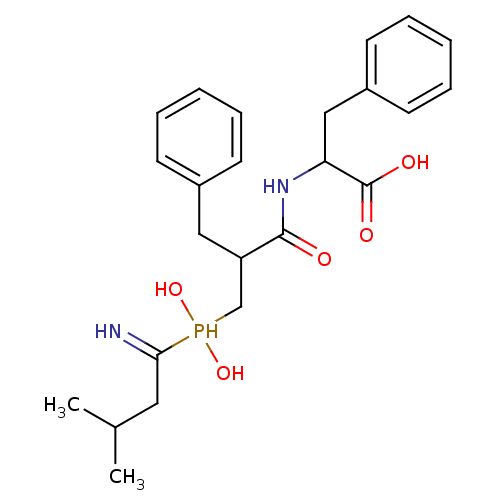
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed]
Chen, H; Roques, BP; Fournié-Zaluski, MC Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg Med Chem Lett9:1511-6 (1999) [PubMed]