| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sucrase-isomaltase, intestinal |
|---|
| Ligand | BDBM50024128 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_206286 |
|---|
| IC50 | 15±n/a nM |
|---|
| Citation |  Horii, S; Fukase, H; Matsuo, T; Kameda, Y; Asano, N; Matsui, K Synthesis and alpha-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J Med Chem29:1038-46 (1986) [PubMed] Horii, S; Fukase, H; Matsuo, T; Kameda, Y; Asano, N; Matsui, K Synthesis and alpha-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J Med Chem29:1038-46 (1986) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sucrase-isomaltase, intestinal |
|---|
| Name: | Sucrase-isomaltase, intestinal |
|---|
| Synonyms: | Alpha glucosidase | Isomaltase | SI | SUIS_HUMAN | Sucrase | Sucrase-isomaltase | Sucrase-isomaltase, intestinal |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 209423.23 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1435477 |
|---|
| Residue: | 1827 |
|---|
| Sequence: | MARKKFSGLEISLIVLFVIVTIIAIALIVVLATKTPAVDEISDSTSTPATTRVTTNPSDS
GKCPNVLNDPVNVRINCIPEQFPTEGICAQRGCCWRPWNDSLIPWCFFVDNHGYNVQDMT
TTSIGVEAKLNRIPSPTLFGNDINSVLFTTQNQTPNRFRFKITDPNNRRYEVPHQYVKEF
TGPTVSDTLYDVKVAQNPFSIQVIRKSNGKTLFDTSIGPLVYSDQYLQISTRLPSDYIYG
IGEQVHKRFRHDLSWKTWPIFTRDQLPGDNNNNLYGHQTFFMCIEDTSGKSFGVFLMNSN
AMEIFIQPTPIVTYRVTGGILDFYILLGDTPEQVVQQYQQLVGLPAMPAYWNLGFQLSRW
NYKSLDVVKEVVRRNREAGIPFDTQVTDIDYMEDKKDFTYDQVAFNGLPQFVQDLHDHGQ
KYVIILDPAISIGRRANGTTYATYERGNTQHVWINESDGSTPIIGEVWPGLTVYPDFTNP
NCIDWWANECSIFHQEVQYDGLWIDMNEVSSFIQGSTKGCNVNKLNYPPFTPDILDKLMY
SKTICMDAVQNWGKQYDVHSLYGYSMAIATEQAVQKVFPNKRSFILTRSTFAGSGRHAAH
WLGDNTASWEQMEWSITGMLEFSLFGIPLVGADICGFVAETTEELCRRWMQLGAFYPFSR
NHNSDGYEHQDPAFFGQNSLLVKSSRQYLTIRYTLLPFLYTLFYKAHVFGETVARPVLHE
FYEDTNSWIEDTEFLWGPALLITPVLKQGADTVSAYIPDAIWYDYESGAKRPWRKQRVDM
YLPADKIGLHLRGGYIIPIQEPDVTTTASRKNPLGLIVALGENNTAKGDFFWDDGETKDT
IQNGNYILYTFSVSNNTLDIVCTHSSYQEGTTLAFQTVKILGLTDSVTEVRVAENNQPMN
AHSNFTYDASNQVLLIADLKLNLGRNFSVQWNQIFSENERFNCYPDADLATEQKCTQRGC
VWRTGSSLSKAPECYFPRQDNSYSVNSARYSSMGITADLQLNTANARIKLPSDPISTLRV
EVKYHKNDMLQFKIYDPQKKRYEVPVPLNIPTTPISTYEDRLYDVEIKENPFGIQIRRRS
SGRVIWDSWLPGFAFNDQFIQISTRLPSEYIYGFGEVEHTAFKRDLNWNTWGMFTRDQPP
GYKLNSYGFHPYYMALEEEGNAHGVFLLNSNAMDVTFQPTPALTYRTVGGILDFYMFLGP
TPEVATKQYHEVIGHPVMPAYWALGFQLCRYGYANTSEVRELYDAMVAANIPYDVQYTDI
DYMERQLDFTIGEAFQDLPQFVDKIRGEGMRYIIILDPAISGNETKTYPAFERGQQNDVF
VKWPNTNDICWAKVWPDLPNITIDKTLTEDEAVNASRAHVAFPDFFRTSTAEWWAREIVD
FYNEKMKFDGLWIDMNEPSSFVNGTTTNQCRNDELNYPPYFPELTKRTDGLHFRTICMEA
EQILSDGTSVLHYDVHNLYGWSQMKPTHDALQKTTGKRGIVISRSTYPTSGRWGGHWLGD
NYARWDNMDKSIIGMMEFSLFGMSYTGADICGFFNNSEYHLCTRWMQLGAFYPYSRNHNI
ANTRRQDPASWNETFAEMSRNILNIRYTLLPYFYTQMHEIHANGGTVIRPLLHEFFDEKP
TWDIFKQFLWGPAFMVTPVLEPYVQTVNAYVPNARWFDYHTGKDIGVRGQFQTFNASYDT
INLHVRGGHILPCQEPAQNTFYSRQKHMKLIVAADDNQMAQGSLFWDDGESIDTYERDLY
LSVQFNLNQTTLTSTILKRGYINKSETRLGSLHVWGKGTTPVNAVTLTYNGNKNSLPFNE
DTTNMILRIDLTTHNVTLEEPIEINWS
|
|
|
|---|
| BDBM50024128 |
|---|
| n/a |
|---|
| Name | BDBM50024128 |
|---|
| Synonyms: | 5-(4-Bromo-benzylamino)-1-hydroxymethyl-cyclohexane-1,2,3,4-tetraol | CHEMBL3349435 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H20BrNO5 |
|---|
| Mol. Mass. | 362.216 |
|---|
| SMILES | OC[C@@]1(O)C[C@H](NCc2ccc(Br)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| |
|---|
| Structure | 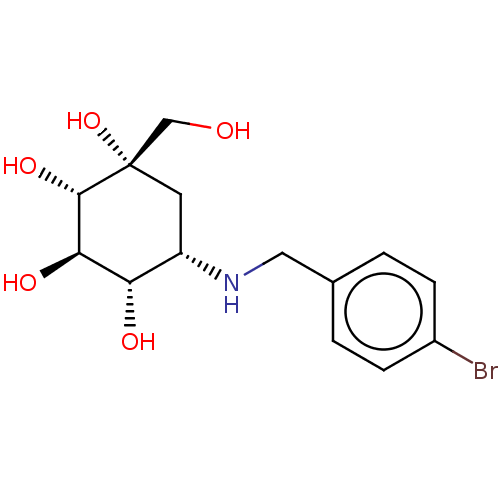
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Horii, S; Fukase, H; Matsuo, T; Kameda, Y; Asano, N; Matsui, K Synthesis and alpha-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J Med Chem29:1038-46 (1986) [PubMed]
Horii, S; Fukase, H; Matsuo, T; Kameda, Y; Asano, N; Matsui, K Synthesis and alpha-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J Med Chem29:1038-46 (1986) [PubMed]