 Found 429 hits with Last Name = 'matsui' and Initial = 'k'
Found 429 hits with Last Name = 'matsui' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50065257

((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...)Show SMILES C[C@@H]1N[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C7H15NO4/c1-3-5(10)7(12)6(11)4(2-9)8-3/h3-12H,2H2,1H3/t3-,4+,5+,6+,7+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50065258
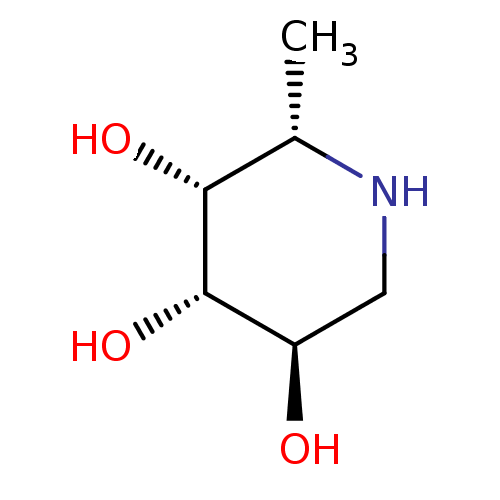
((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...)Show InChI InChI=1S/C6H13NO3/c1-3-5(9)6(10)4(8)2-7-3/h3-10H,2H2,1H3/t3-,4+,5+,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50408432
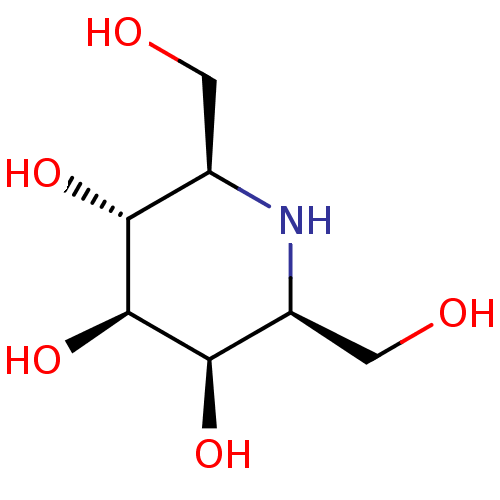
(CHEMBL2115215)Show SMILES OC[C@H]1N[C@@H](CO)[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4+,5-,6-,7+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against alpha-glucosidase |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50065259

((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4-,5-,6-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50408431
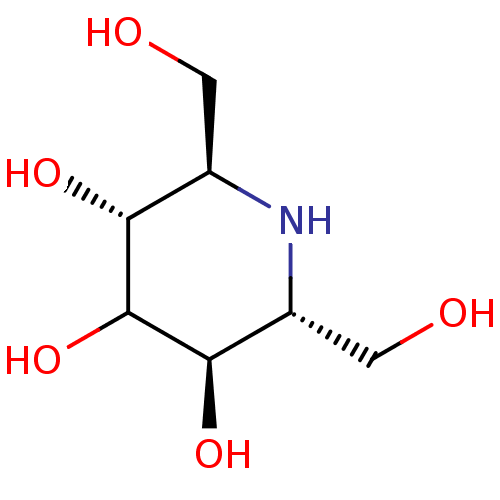
(CHEMBL2114210)Show InChI InChI=1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4-,5-,6-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against alpha-glucosidase |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50065255

((R)-2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol |...)Show InChI InChI=1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3?,4?,5-,6?,7?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against alpha-glucosidase |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM50016703
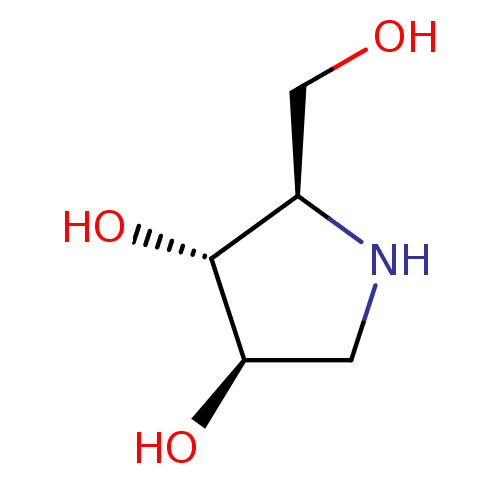
(2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM50031480
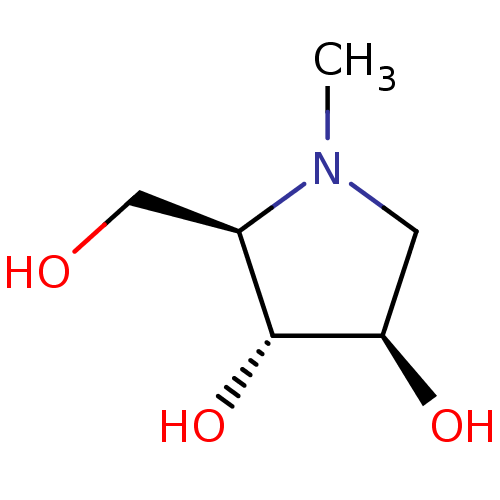
((2R,3R,4R)-2-Hydroxymethyl-1-methyl-pyrrolidine-3,...)Show InChI InChI=1S/C6H13NO3/c1-7-2-5(9)6(10)4(7)3-8/h4-6,8-10H,2-3H2,1H3/t4-,5-,6-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Alpha-mannosidase 2
(Rattus norvegicus) | BDBM50031484
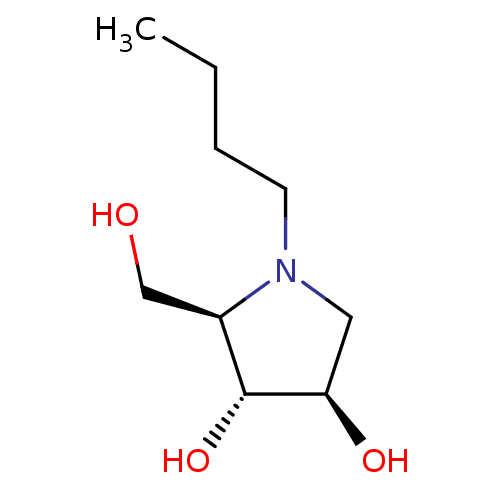
((2R,3R,4R)-1-Butyl-2-hydroxymethyl-pyrrolidine-3,4...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-8(12)9(13)7(10)6-11/h7-9,11-13H,2-6H2,1H3/t7-,8-,9-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Competitive Inhibitory activity against Golgi Alpha-mannosidase II |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Neutral alpha-glucosidase AB
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Tested for competitive inhibition of golgi alpha mannosidase II |
J Med Chem 37: 3701-6 (1994)
BindingDB Entry DOI: 10.7270/Q2ZC83H3 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024139

(1-Hydroxymethyl-5-phenethylamino-cyclohexane-1,2,3...)Show SMILES OC[C@@]1(O)C[C@H](NCCc2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C15H23NO5/c17-9-15(21)8-11(12(18)13(19)14(15)20)16-7-6-10-4-2-1-3-5-10/h1-5,11-14,16-21H,6-9H2/t11-,12?,13-,14-,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024120

(1-Hydroxymethyl-5-[(thiophen-2-ylmethyl)-amino]-cy...)Show SMILES OC[C@@]1(O)C[C@H](NCc2cccs2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C12H19NO5S/c14-6-12(18)4-8(9(15)10(16)11(12)17)13-5-7-2-1-3-19-7/h1-3,8-11,13-18H,4-6H2/t8-,9?,10-,11-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50226273
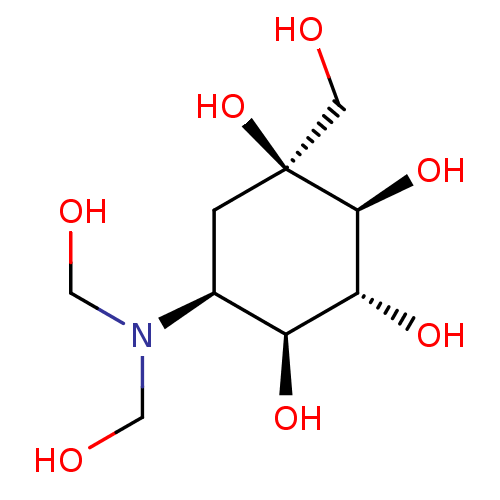
(CHEMBL3349431)Show SMILES OCN(CO)[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H19NO7/c11-2-9(17)1-5(10(3-12)4-13)6(14)7(15)8(9)16/h5-8,11-17H,1-4H2/t5-,6-,7+,8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50405397
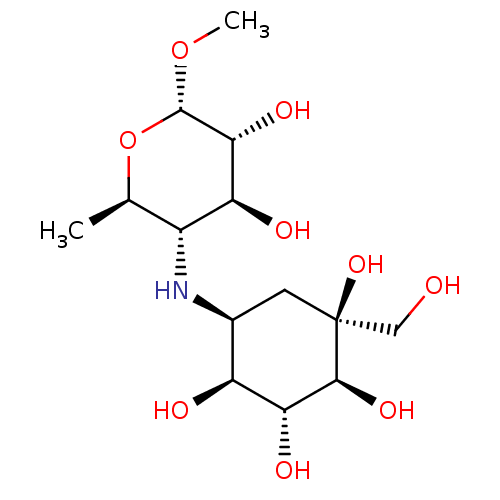
(CHEMBL2051983)Show SMILES CO[C@H]1O[C@H](C)[C@@H](N[C@H]2C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO9/c1-5-7(9(18)11(20)13(23-2)24-5)15-6-3-14(22,4-16)12(21)10(19)8(6)17/h5-13,15-22H,3-4H2,1-2H3/t5-,6+,7-,8+,9+,10-,11-,12+,13+,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024119
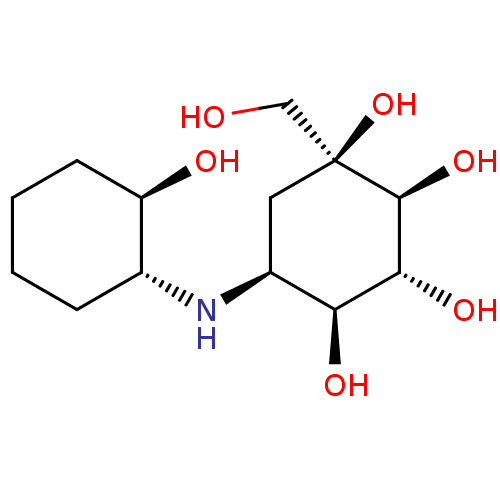
(5-(2-Hydroxy-cyclohexylamino)-1-hydroxymethyl-cycl...)Show SMILES OC[C@@]1(O)C[C@H](N[C@@H]2CCCC[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H25NO6/c15-6-13(20)5-8(10(17)11(18)12(13)19)14-7-3-1-2-4-9(7)16/h7-12,14-20H,1-6H2/t7-,8-,9-,10?,11-,12-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024137
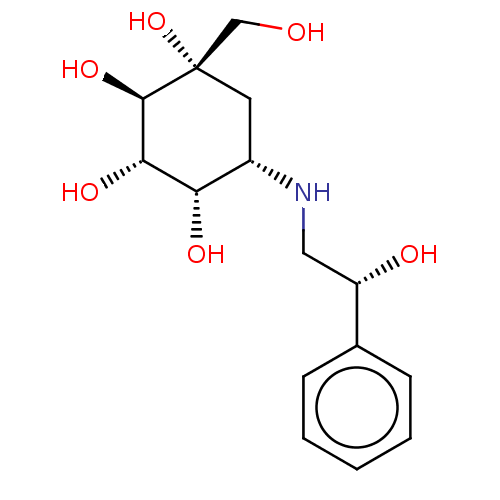
(1-Hydroxymethyl-5-(2-hydroxy-2-phenyl-ethylamino)-...)Show SMILES [H][C@](O)(CN[C@H]1C[C@](O)(CO)[C@H](O)[C@@H](O)[C@H]1O)c1ccccc1 Show InChI InChI=1S/C15H23NO6/c17-8-15(22)6-10(12(19)13(20)14(15)21)16-7-11(18)9-4-2-1-3-5-9/h1-5,10-14,16-22H,6-8H2/t10-,11-,12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50024137

(1-Hydroxymethyl-5-(2-hydroxy-2-phenyl-ethylamino)-...)Show SMILES [H][C@](O)(CN[C@H]1C[C@](O)(CO)[C@H](O)[C@@H](O)[C@H]1O)c1ccccc1 Show InChI InChI=1S/C15H23NO6/c17-8-15(22)6-10(12(19)13(20)14(15)21)16-7-11(18)9-4-2-1-3-5-9/h1-5,10-14,16-22H,6-8H2/t10-,11-,12-,13-,14+,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50024119

(5-(2-Hydroxy-cyclohexylamino)-1-hydroxymethyl-cycl...)Show SMILES OC[C@@]1(O)C[C@H](N[C@@H]2CCCC[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H25NO6/c15-6-13(20)5-8(10(17)11(18)12(13)19)14-7-3-1-2-4-9(7)16/h7-12,14-20H,1-6H2/t7-,8-,9-,10?,11-,12-,13?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024136
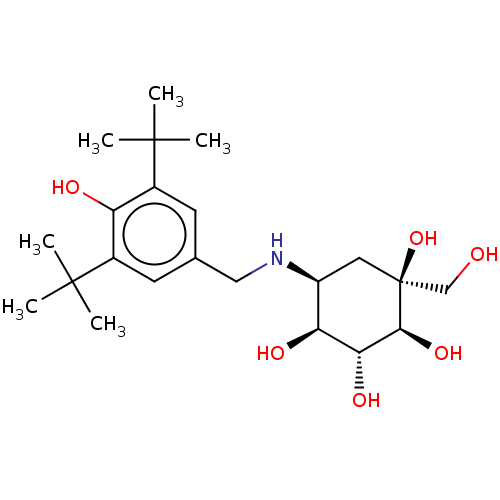
(5-(3,5-Di-tert-butyl-4-hydroxy-benzylamino)-1-hydr...)Show SMILES CC(C)(C)c1cc(CN[C@H]2C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]2O)cc(c1O)C(C)(C)C |r| Show InChI InChI=1S/C22H37NO6/c1-20(2,3)13-7-12(8-14(16(13)25)21(4,5)6)10-23-15-9-22(29,11-24)19(28)18(27)17(15)26/h7-8,15,17-19,23-29H,9-11H2,1-6H3/t15-,17?,18-,19-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405395
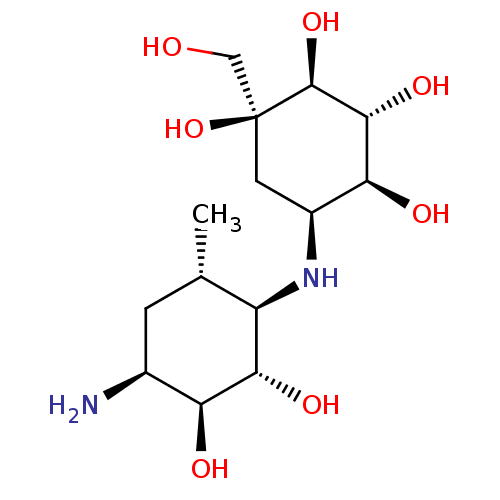
(CHEMBL2051761)Show SMILES C[C@H]1C[C@H](N)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H28N2O7/c1-5-2-6(15)9(18)11(20)8(5)16-7-3-14(23,4-17)13(22)12(21)10(7)19/h5-13,16-23H,2-4,15H2,1H3/t5-,6-,7-,8+,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024114

(5-(Cyclohexylmethyl-amino)-1-hydroxymethyl-cyclohe...)Show SMILES OC[C@@]1(O)C[C@H](NCC2CCCCC2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C14H27NO5/c16-8-14(20)6-10(11(17)12(18)13(14)19)15-7-9-4-2-1-3-5-9/h9-13,15-20H,1-8H2/t10-,11?,12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405397

(CHEMBL2051983)Show SMILES CO[C@H]1O[C@H](C)[C@@H](N[C@H]2C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO9/c1-5-7(9(18)11(20)13(23-2)24-5)15-6-3-14(22,4-16)12(21)10(19)8(6)17/h5-13,15-22H,3-4H2,1-2H3/t5-,6+,7-,8+,9+,10-,11-,12+,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024118
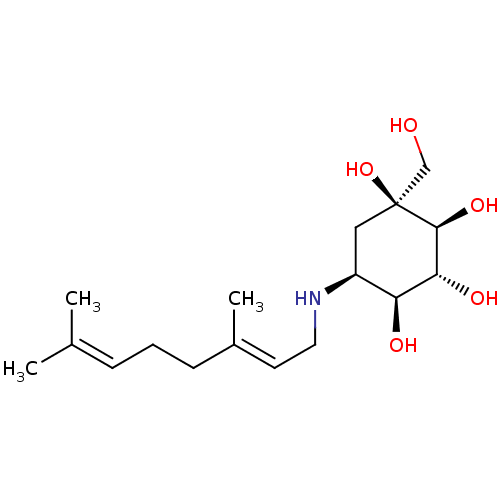
(5-(3,7-Dimethyl-octa-2,6-dienylamino)-1-hydroxymet...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#7]-[#6@H]-1-[#6][C@]([#8])([#6]-[#8])[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-1-[#8] |r| Show InChI InChI=1S/C17H31NO5/c1-11(2)5-4-6-12(3)7-8-18-13-9-17(23,10-19)16(22)15(21)14(13)20/h5,7,13-16,18-23H,4,6,8-10H2,1-3H3/b12-7+/t13-,14?,15-,16-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50024124
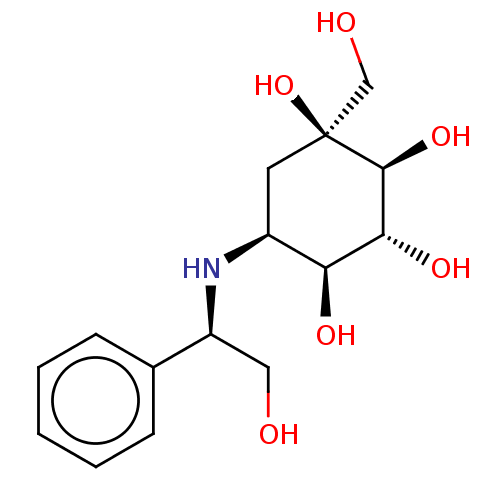
(1-Hydroxymethyl-5-(2-hydroxy-1-phenyl-ethylamino)-...)Show SMILES [H][C@@](CO)(N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O)c1ccccc1 Show InChI InChI=1S/C15H23NO6/c17-7-11(9-4-2-1-3-5-9)16-10-6-15(22,8-18)14(21)13(20)12(10)19/h1-5,10-14,16-22H,6-8H2/t10-,11-,12-,13+,14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50170537
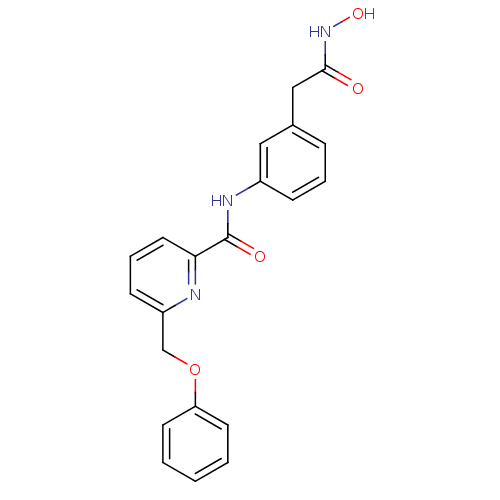
(6-Phenoxymethyl-pyridine-2-carboxylic acid (3-hydr...)Show SMILES ONC(=O)Cc1cccc(NC(=O)c2cccc(COc3ccccc3)n2)c1 Show InChI InChI=1S/C21H19N3O4/c25-20(24-27)13-15-6-4-7-16(12-15)23-21(26)19-11-5-8-17(22-19)14-28-18-9-2-1-3-10-18/h1-12,27H,13-14H2,(H,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Phosphodiesterase type 5 |
Bioorg Med Chem Lett 15: 4085-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.016
BindingDB Entry DOI: 10.7270/Q22B8XKH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024124

(1-Hydroxymethyl-5-(2-hydroxy-1-phenyl-ethylamino)-...)Show SMILES [H][C@@](CO)(N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O)c1ccccc1 Show InChI InChI=1S/C15H23NO6/c17-7-11(9-4-2-1-3-5-9)16-10-6-15(22,8-18)14(21)13(20)12(10)19/h1-5,10-14,16-22H,6-8H2/t10-,11-,12-,13+,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024133

(1-Hydroxymethyl-5-(3-phenyl-allylamino)-cyclohexan...)Show SMILES OC[C@@]1(O)C[C@H](NC\C=C\c2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C16H23NO5/c18-10-16(22)9-12(13(19)14(20)15(16)21)17-8-4-7-11-5-2-1-3-6-11/h1-7,12-15,17-22H,8-10H2/b7-4+/t12-,13?,14-,15-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50226273

(CHEMBL3349431)Show SMILES OCN(CO)[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H19NO7/c11-2-9(17)1-5(10(3-12)4-13)6(14)7(15)8(9)16/h5-8,11-17H,1-4H2/t5-,6-,7+,8-,9-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024128
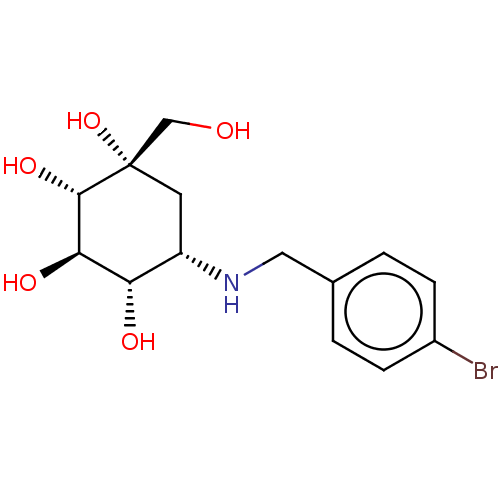
(5-(4-Bromo-benzylamino)-1-hydroxymethyl-cyclohexan...)Show SMILES OC[C@@]1(O)C[C@H](NCc2ccc(Br)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C14H20BrNO5/c15-9-3-1-8(2-4-9)6-16-10-5-14(21,7-17)13(20)12(19)11(10)18/h1-4,10-13,16-21H,5-7H2/t10-,11?,12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024126
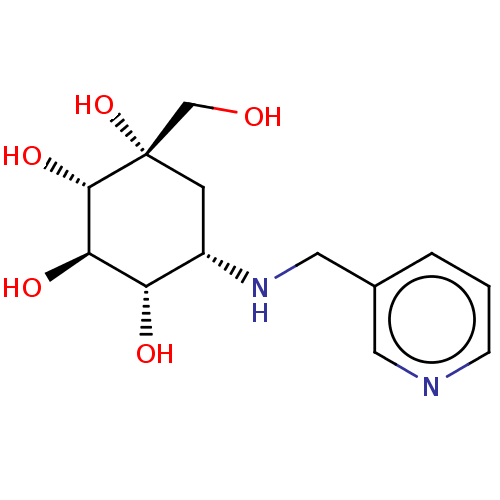
(1-Hydroxymethyl-5-[(pyridin-3-ylmethyl)-amino]-cyc...)Show SMILES OC[C@@]1(O)C[C@H](NCc2cccnc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H20N2O5/c16-7-13(20)4-9(10(17)11(18)12(13)19)15-6-8-2-1-3-14-5-8/h1-3,5,9-12,15-20H,4,6-7H2/t9-,10?,11-,12-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024117

(5-Cyclohexylamino-1-hydroxymethyl-cyclohexane-1,2,...)Show SMILES OC[C@@]1(O)C[C@H](NC2CCCCC2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H25NO5/c15-7-13(19)6-9(10(16)11(17)12(13)18)14-8-4-2-1-3-5-8/h8-12,14-19H,1-7H2/t9-,10?,11-,12-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50170540

(6-(4-Acetyl-2-ethyl-5-hydroxy-phenoxymethyl)-pyrid...)Show SMILES CCc1cc(C(C)=O)c(O)cc1OCc1cccc(n1)C(=O)NCCCCCCCCCC(=O)NO Show InChI InChI=1S/C27H37N3O6/c1-3-20-16-22(19(2)31)24(32)17-25(20)36-18-21-12-11-13-23(29-21)27(34)28-15-10-8-6-4-5-7-9-14-26(33)30-35/h11-13,16-17,32,35H,3-10,14-15,18H2,1-2H3,(H,28,34)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Phosphodiesterase type 5 |
Bioorg Med Chem Lett 15: 4085-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.016
BindingDB Entry DOI: 10.7270/Q22B8XKH |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50065252

(2,6-Bis-hydroxymethyl-1-methyl-piperidine-3,4,5-tr...)Show SMILES CN1[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C8H17NO5/c1-9-4(2-10)6(12)8(14)7(13)5(9)3-11/h4-8,10-14H,2-3H2,1H3/t4-,5-,6-,7+,8+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against alpha-glucosidase of rat intestinal sucrase by colorimetric assay using the D-glucose oxidase-peroxidase method |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50170480
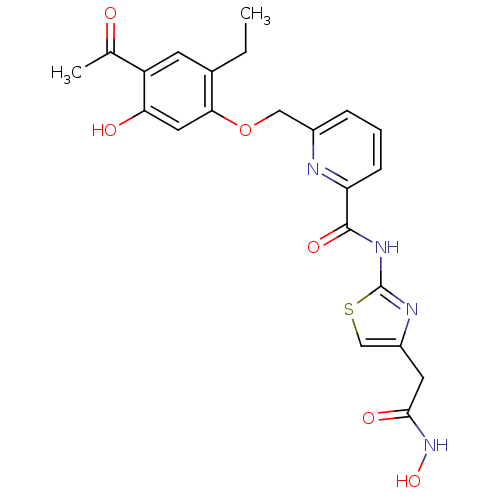
(6-(4-Acetyl-2-ethyl-5-hydroxy-phenoxymethyl)-pyrid...)Show SMILES CCc1cc(C(C)=O)c(O)cc1OCc1cccc(n1)C(=O)Nc1nc(CC(=O)NO)cs1 Show InChI InChI=1S/C22H22N4O6S/c1-3-13-7-16(12(2)27)18(28)9-19(13)32-10-14-5-4-6-17(23-14)21(30)25-22-24-15(11-33-22)8-20(29)26-31/h4-7,9,11,28,31H,3,8,10H2,1-2H3,(H,26,29)(H,24,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Phosphodiesterase type 5 |
Bioorg Med Chem Lett 15: 4085-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.016
BindingDB Entry DOI: 10.7270/Q22B8XKH |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50405395

(CHEMBL2051761)Show SMILES C[C@H]1C[C@H](N)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H28N2O7/c1-5-2-6(15)9(18)11(20)8(5)16-7-3-14(23,4-17)13(22)12(21)10(7)19/h5-13,16-23H,2-4,15H2,1H3/t5-,6-,7-,8+,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405389
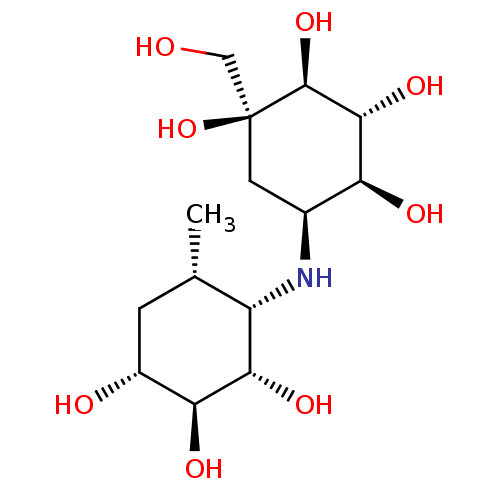
(CHEMBL2051982)Show SMILES C[C@H]1C[C@@H](O)[C@H](O)[C@@H](O)[C@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO8/c1-5-2-7(17)10(19)11(20)8(5)15-6-3-14(23,4-16)13(22)12(21)9(6)18/h5-13,15-23H,2-4H2,1H3/t5-,6-,7+,8-,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405396

(CHEMBL2051762)Show SMILES C[C@H]1C[C@H](O)[C@H](O)[C@@H](O)[C@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO8/c1-5-2-7(17)10(19)11(20)8(5)15-6-3-14(23,4-16)13(22)12(21)9(6)18/h5-13,15-23H,2-4H2,1H3/t5-,6-,7-,8-,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50259956
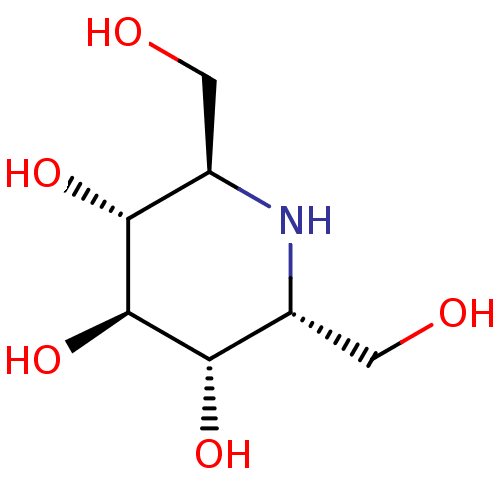
(2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...)Show SMILES OC[C@H]1N[C@H](CO)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4-,5-,6+,7+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against alpha-glucosidase of rice by colorimetric assay using the D-glucose oxidase-peroxidase method |
J Med Chem 41: 2565-71 (1998)
Article DOI: 10.1021/jm970836l
BindingDB Entry DOI: 10.7270/Q25D8SHK |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50170475

(6-Phenoxymethyl-pyridine-2-carboxylic acid (6-hydr...)Show SMILES ONC(=O)Cc1cccc(NC(=O)c2cccc(COc3ccccc3)n2)n1 Show InChI InChI=1S/C20H18N4O4/c25-19(24-27)12-14-6-5-11-18(22-14)23-20(26)17-10-4-7-15(21-17)13-28-16-8-2-1-3-9-16/h1-11,27H,12-13H2,(H,24,25)(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Phosphodiesterase type 5 |
Bioorg Med Chem Lett 15: 4085-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.016
BindingDB Entry DOI: 10.7270/Q22B8XKH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of Sucrase in rat intestinal brush border membranes by D-glucose oxidase-peroxidase method |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50170473
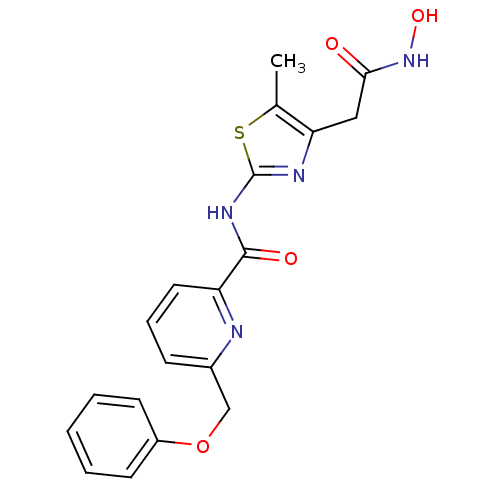
(6-Phenoxymethyl-pyridine-2-carboxylic acid (4-hydr...)Show SMILES Cc1sc(NC(=O)c2cccc(COc3ccccc3)n2)nc1CC(=O)NO Show InChI InChI=1S/C19H18N4O4S/c1-12-16(10-17(24)23-26)21-19(28-12)22-18(25)15-9-5-6-13(20-15)11-27-14-7-3-2-4-8-14/h2-9,26H,10-11H2,1H3,(H,23,24)(H,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Phosphodiesterase type 5 |
Bioorg Med Chem Lett 15: 4085-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.016
BindingDB Entry DOI: 10.7270/Q22B8XKH |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of Sucrase in rat intestinal brush border membranes by D-glucose oxidase-peroxidase method |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A/1B/1C
(Homo sapiens (Human)) | BDBM50170491
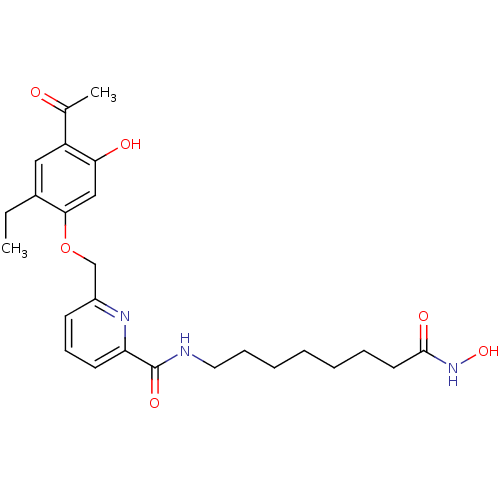
(6-(4-Acetyl-2-ethyl-5-hydroxy-phenoxymethyl)-pyrid...)Show SMILES CCc1cc(C(C)=O)c(O)cc1OCc1cccc(n1)C(=O)NCCCCCCCC(=O)NO Show InChI InChI=1S/C25H33N3O6/c1-3-18-14-20(17(2)29)22(30)15-23(18)34-16-19-10-9-11-21(27-19)25(32)26-13-8-6-4-5-7-12-24(31)28-33/h9-11,14-15,30,33H,3-8,12-13,16H2,1-2H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Phosphodiesterase type 1 |
Bioorg Med Chem Lett 15: 4085-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.016
BindingDB Entry DOI: 10.7270/Q22B8XKH |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50024136

(5-(3,5-Di-tert-butyl-4-hydroxy-benzylamino)-1-hydr...)Show SMILES CC(C)(C)c1cc(CN[C@H]2C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]2O)cc(c1O)C(C)(C)C |r| Show InChI InChI=1S/C22H37NO6/c1-20(2,3)13-7-12(8-14(16(13)25)21(4,5)6)10-23-15-9-22(29,11-24)19(28)18(27)17(15)26/h7-8,15,17-19,23-29H,9-11H2,1-6H3/t15-,17?,18-,19-,22?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024129

(5-Amino-1-hydroxymethyl-cyclohexane-1,2,3,4-tetrao...)Show SMILES N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C7H15NO5/c8-3-1-7(13,2-9)6(12)5(11)4(3)10/h3-6,9-13H,1-2,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50405396

(CHEMBL2051762)Show SMILES C[C@H]1C[C@H](O)[C@H](O)[C@@H](O)[C@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO8/c1-5-2-7(17)10(19)11(20)8(5)15-6-3-14(23,4-16)13(22)12(21)9(6)18/h5-13,15-23H,2-4H2,1H3/t5-,6-,7-,8-,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM50405389

(CHEMBL2051982)Show SMILES C[C@H]1C[C@@H](O)[C@H](O)[C@@H](O)[C@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO8/c1-5-2-7(17)10(19)11(20)8(5)15-6-3-14(23,4-16)13(22)12(21)9(6)18/h5-13,15-23H,2-4H2,1H3/t5-,6-,7+,8-,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine maltase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data