

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 8.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280031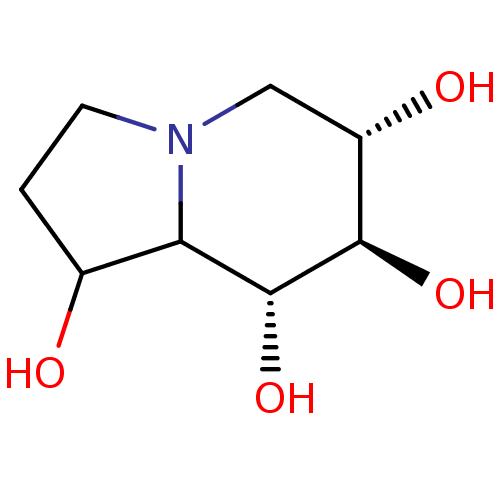 ((6S,7R,8R)-Octahydro-indolizine-1,6,7,8-tetraol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 15 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280029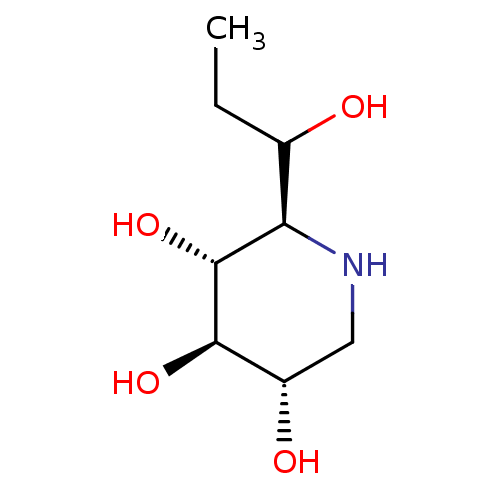 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030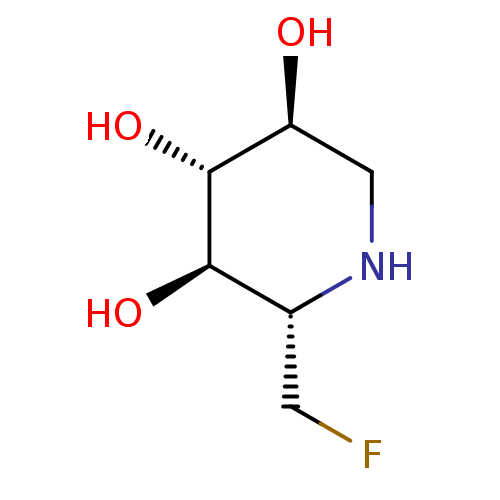 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 6.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on Asp. Wentii beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280029 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on almonds beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Tested for competitive inhibition of golgi alpha mannosidase II | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280031 ((6S,7R,8R)-Octahydro-indolizine-1,6,7,8-tetraol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280032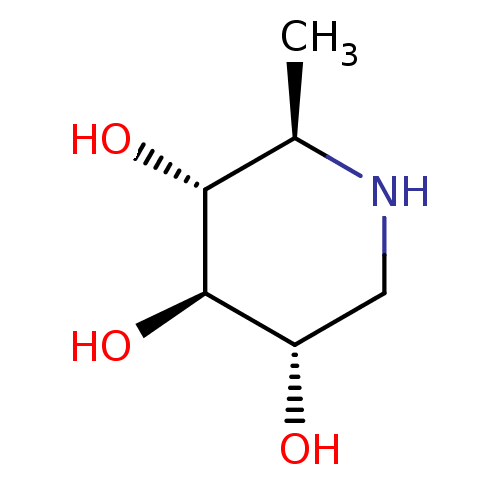 ((2R,3R,4R,5S)-2-Methyl-piperidine-3,4,5-triol | 1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280029 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Tested for competitive inhibition of endoplasmic reticulum alpha-glucosidase II. | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (Golgi I) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50407255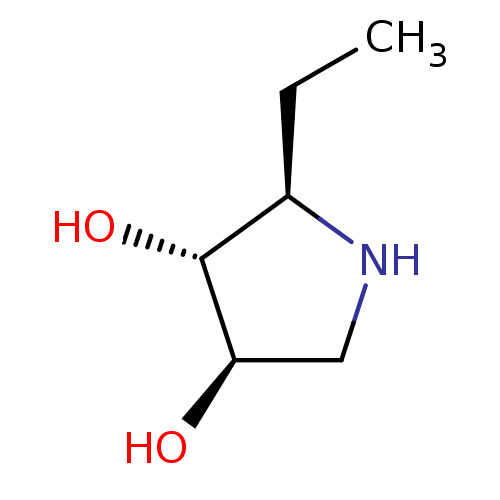 (CHEMBL2114189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (golgi II) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50407255 (CHEMBL2114189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (golgi I) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50407255 (CHEMBL2114189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase was determined in rat epididymis | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50407255 (CHEMBL2114189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of soluble mammalian alpha-mannosidase in rat liver; NI is less than 50 % inhibition at 1000 uM | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50407255 (CHEMBL2114189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (lysosomal) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase was determined in rat epididymis | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of soluble mammalian alpha-mannosidase in rat liver. NI is less than 50 % inhibition at 1000 micro M | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (Golgi II) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (lysosomal) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50403869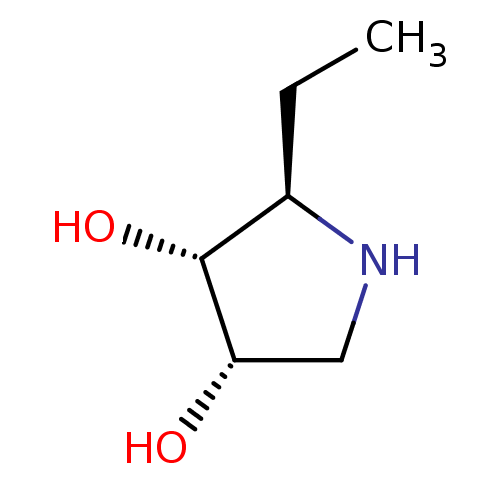 (CHEMBL2115197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (golgi II) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50403869 (CHEMBL2115197) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (lysosomal) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50240725 ((2R,3R,4R)-2-Hydroxymethyl-piperidine-3,4-diol | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Concentration that causes 50% inhibition of mammalian alpha-mannosidase (golgi II) was determined in rat liver | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||