

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065258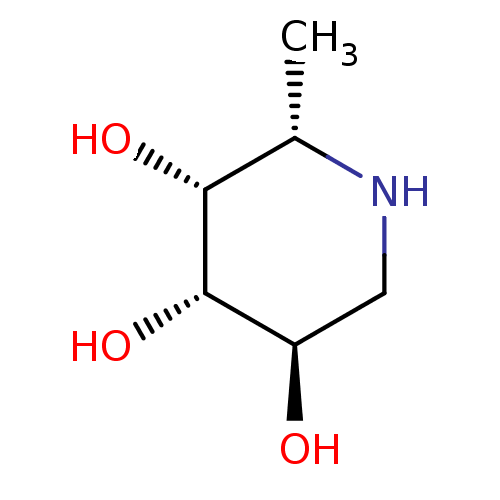 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315250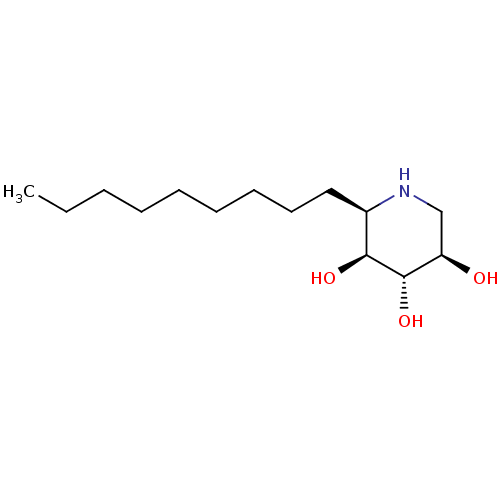 ((2R,3S,4S,5R)-2-nonylpiperidine-3,4,5-triol | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibition of human recombinant beta-glucocerebrosidase assessed as p-nitrophenolate accumulation preincubated for 10 mins before p-nitrophenyl-beta-... | Bioorg Med Chem 18: 2645-50 (2010) Article DOI: 10.1016/j.bmc.2010.02.027 BindingDB Entry DOI: 10.7270/Q24F1QVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065257 ((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically | J Nat Prod 65: 198-202 (2002) BindingDB Entry DOI: 10.7270/Q2ZW1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065257 ((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50242272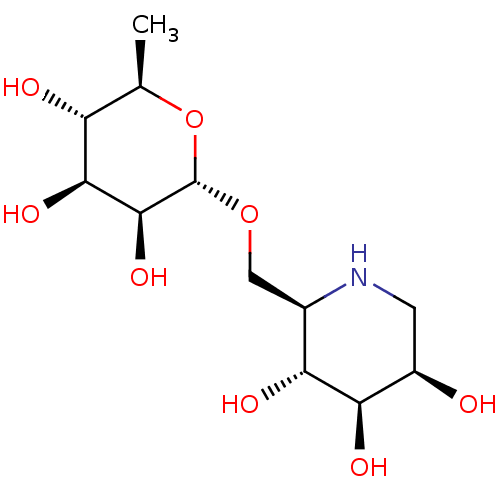 (6-O-alpha-rhamnopyranosyl-DMJ | CHEMBL469655) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically | J Nat Prod 65: 198-202 (2002) BindingDB Entry DOI: 10.7270/Q2ZW1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065257 ((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50163439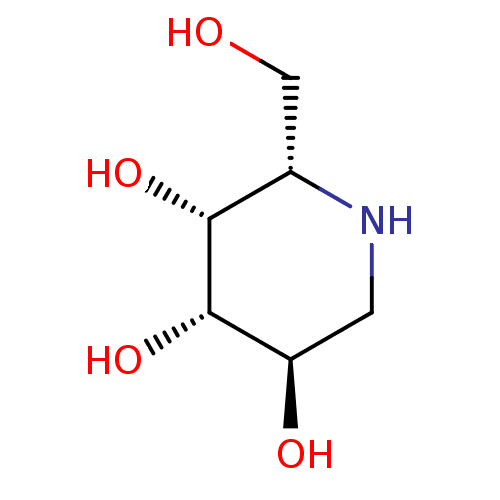 ((2S,3R,4S,5R)-2-Hydroxymethyl-piperidine-3,4,5-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18364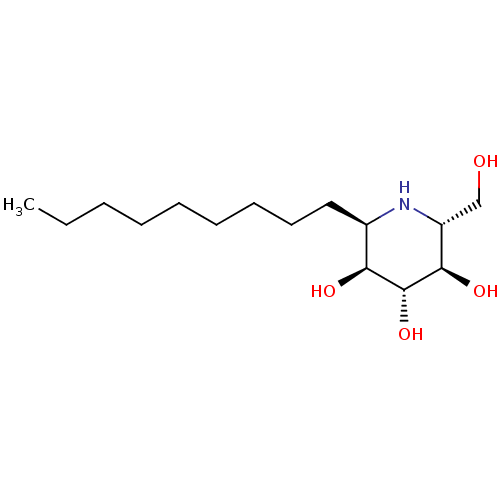 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-nonylpiperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | -39.8 | 270 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18363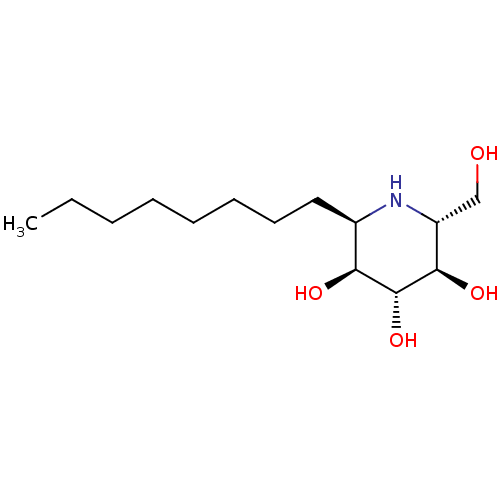 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -38.9 | 500 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358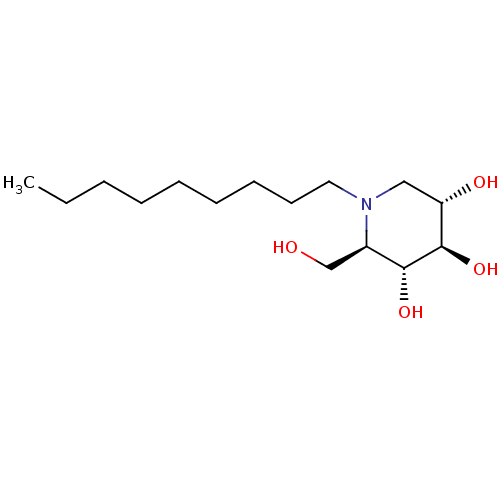 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 300 | -38.7 | 660 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | -37.9 | 820 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50369362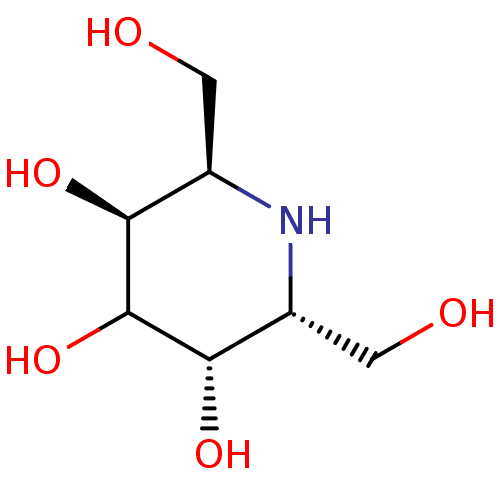 (CHEMBL1169500) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50408432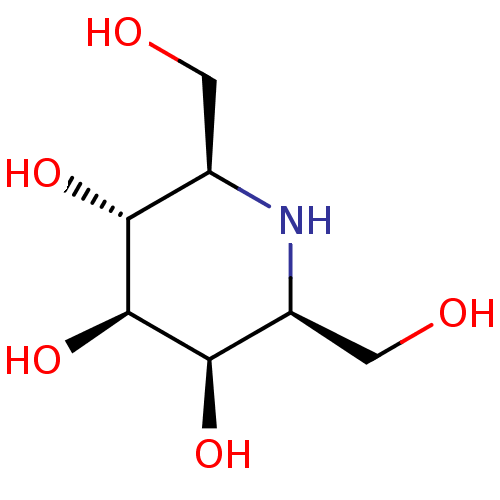 (CHEMBL2115215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50242268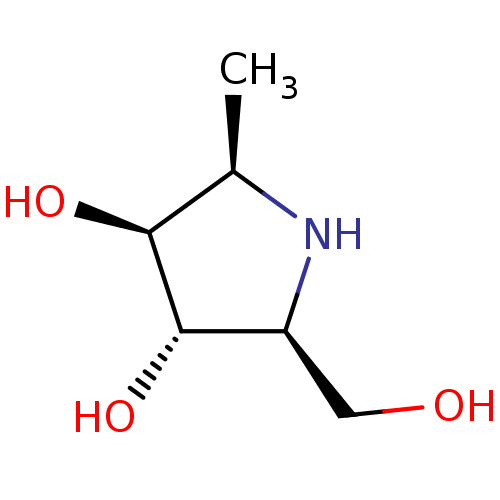 (2,5-Imino-1,2,5-trideoxy-L-glucitol | CHEMBL502230) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically | J Nat Prod 65: 198-202 (2002) BindingDB Entry DOI: 10.7270/Q2ZW1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50241865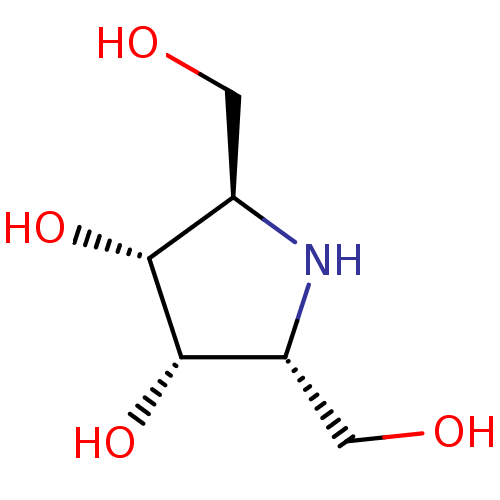 (2,5-dideoxy-2,5-imino-D-altritol | 2R,5R-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of human lysosome alpha-galactosidase by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 3790-4 (2010) Article DOI: 10.1016/j.bmc.2010.04.048 BindingDB Entry DOI: 10.7270/Q2XK8FQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1A4 (Rattus norvegicus) | BDBM18957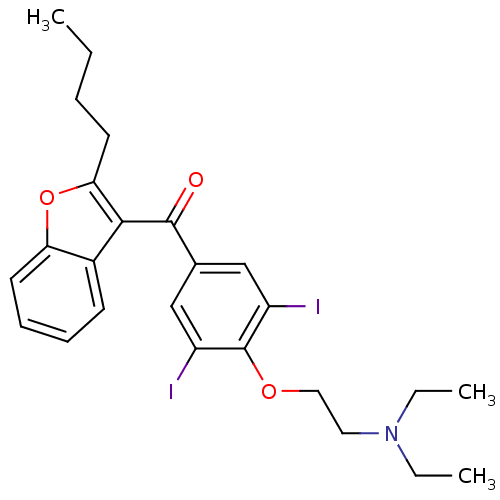 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin uptake in Xenopus laevis oocytes | Endocrinology 142: 2005-12 (2001) Article DOI: 10.1210/endo.142.5.8115 BindingDB Entry DOI: 10.7270/Q2GF0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18362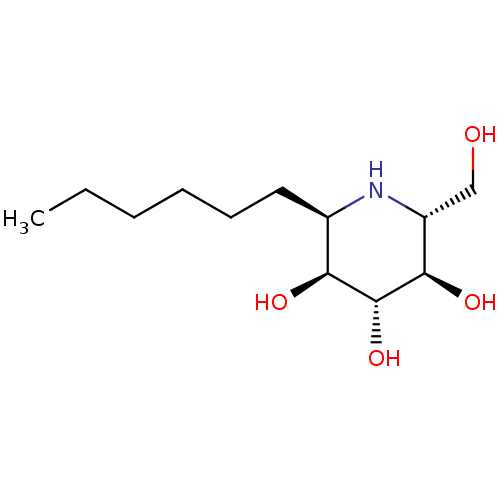 ((2R,3S,4R,5R,6R)-2-hexyl-6-(hydroxymethyl)piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | -33.5 | 4.20E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.50E+3 | -31.2 | 1.30E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50408431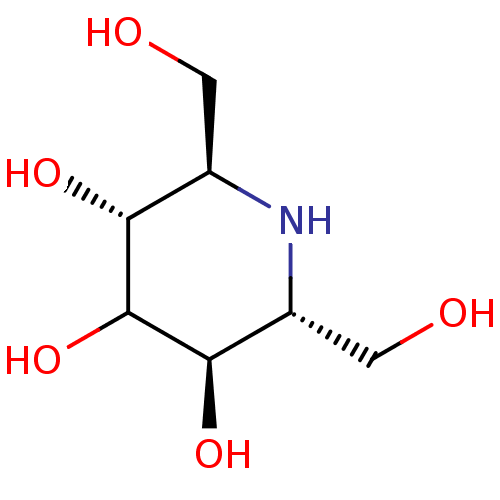 (CHEMBL2114210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50065255 ((R)-2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50163446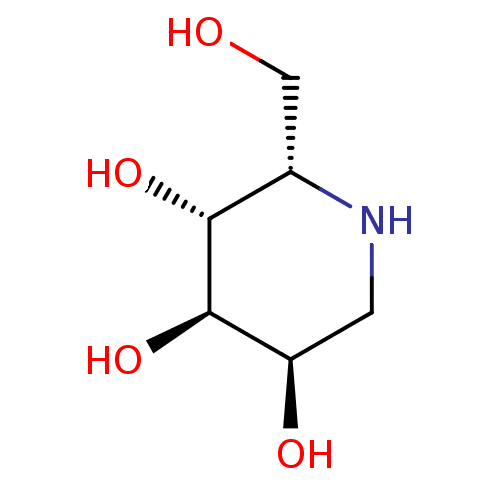 ((2S,3R,4R,5R)-2-Hydroxymethyl-piperidine-3,4,5-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Ki value against bovine alpha-L-fucosidase | J Med Chem 48: 2036-44 (2005) Article DOI: 10.1021/jm0495881 BindingDB Entry DOI: 10.7270/Q2DF6S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50016703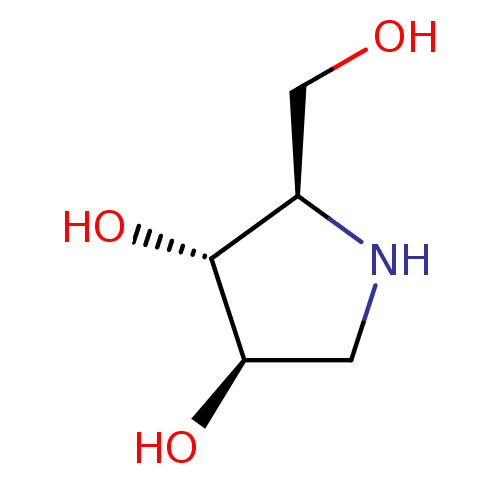 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18353 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50031480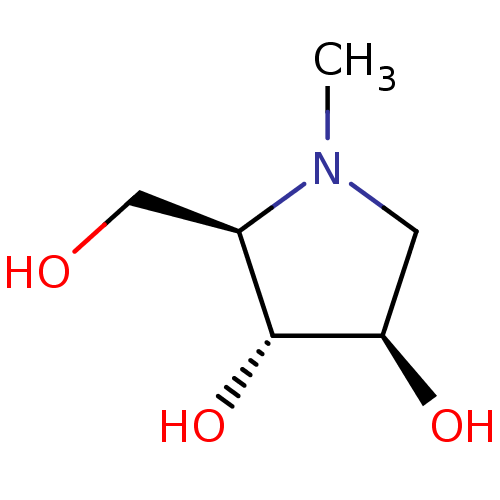 ((2R,3R,4R)-2-Hydroxymethyl-1-methyl-pyrrolidine-3,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.90E+4 | -24.4 | 2.40E+5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18361 ((2R,3S,4R,5R,6R)-2-butyl-6-(hydroxymethyl)piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+5 | -23.5 | 1.00E+5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.16E+5 | -23.4 | 2.70E+5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50031484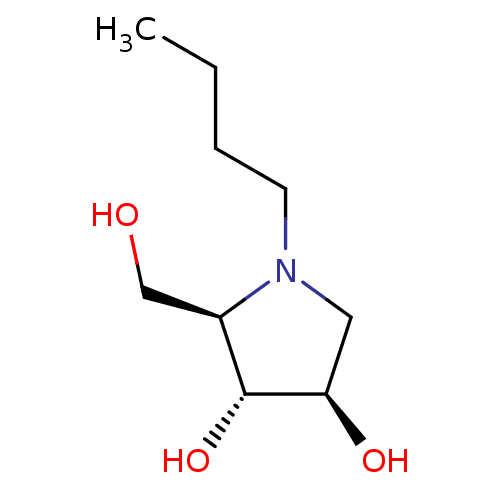 ((2R,3R,4R)-1-Butyl-2-hydroxymethyl-pyrrolidine-3,4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Tested for competitive inhibition of golgi alpha mannosidase II | J Med Chem 37: 3701-6 (1994) BindingDB Entry DOI: 10.7270/Q2ZC83H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024139 (1-Hydroxymethyl-5-phenethylamino-cyclohexane-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50163440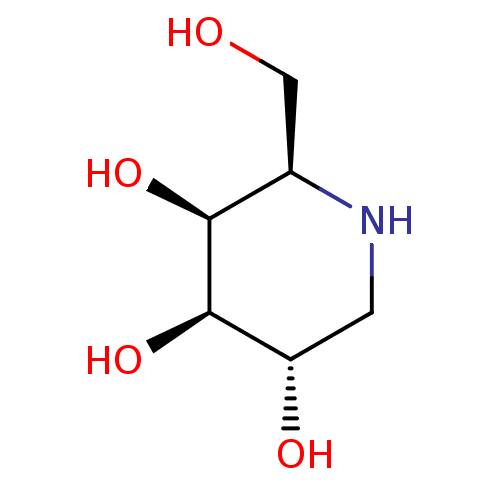 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of coffee bean alpha-galactosidase assessed as p-nitrophenol release at pH 6.5 by spectrometric analysis | Bioorg Med Chem 18: 3790-4 (2010) Article DOI: 10.1016/j.bmc.2010.04.048 BindingDB Entry DOI: 10.7270/Q2XK8FQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024120 (1-Hydroxymethyl-5-[(thiophen-2-ylmethyl)-amino]-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156360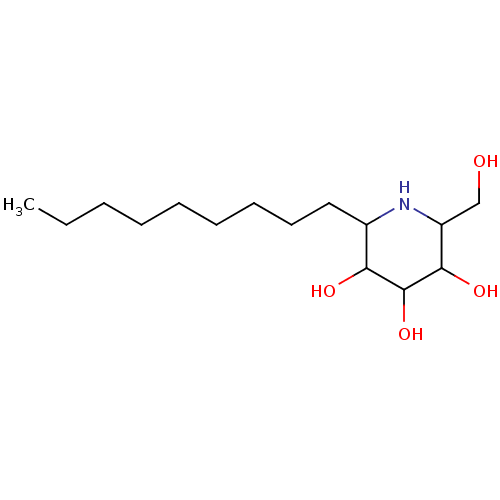 (2-Hydroxymethyl-6-nonyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50226273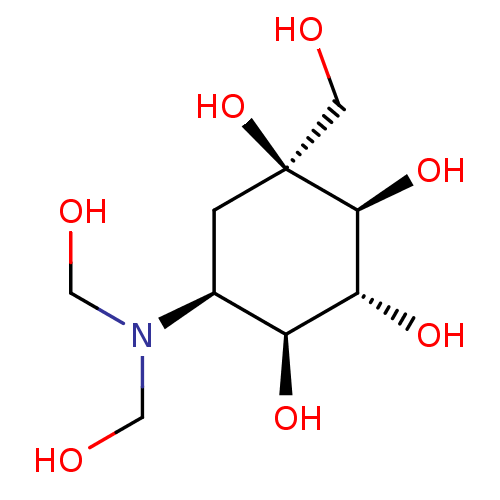 (CHEMBL3349431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50405397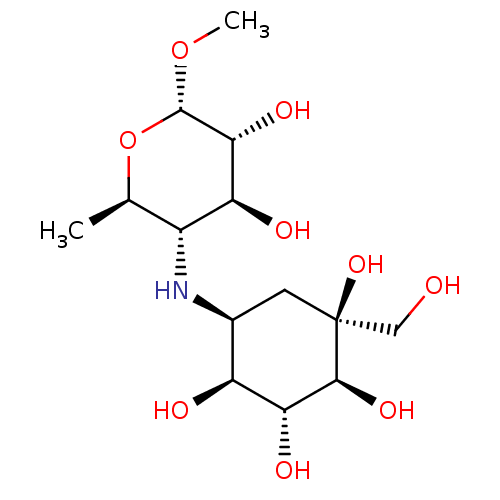 (CHEMBL2051983) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against porcine maltase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024119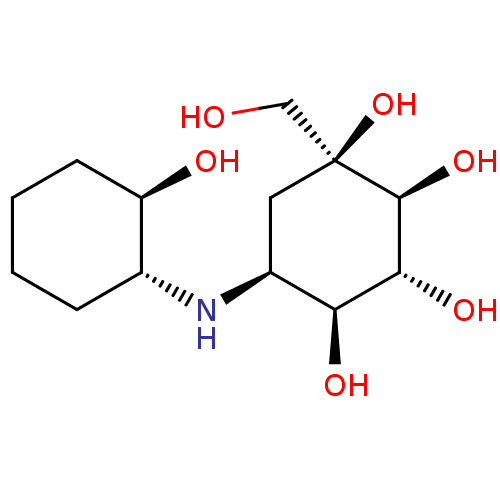 (5-(2-Hydroxy-cyclohexylamino)-1-hydroxymethyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50024137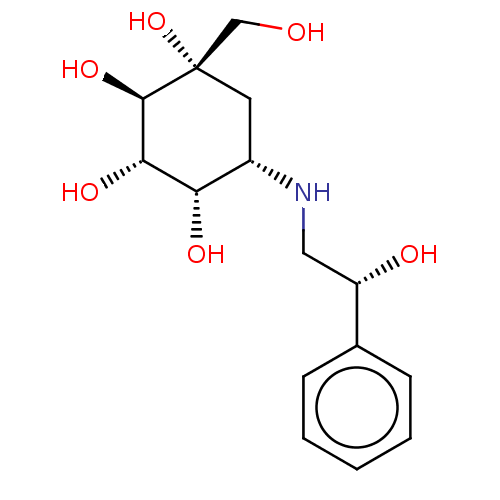 (1-Hydroxymethyl-5-(2-hydroxy-2-phenyl-ethylamino)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against porcine maltase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024137 (1-Hydroxymethyl-5-(2-hydroxy-2-phenyl-ethylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50024119 (5-(2-Hydroxy-cyclohexylamino)-1-hydroxymethyl-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against porcine maltase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024136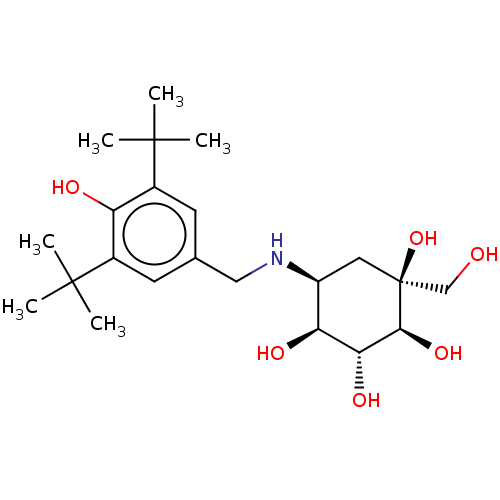 (5-(3,5-Di-tert-butyl-4-hydroxy-benzylamino)-1-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50405395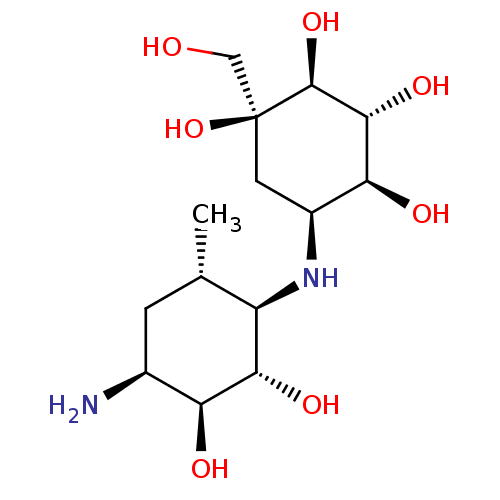 (CHEMBL2051761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024114 (5-(Cyclohexylmethyl-amino)-1-hydroxymethyl-cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50405397 (CHEMBL2051983) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024118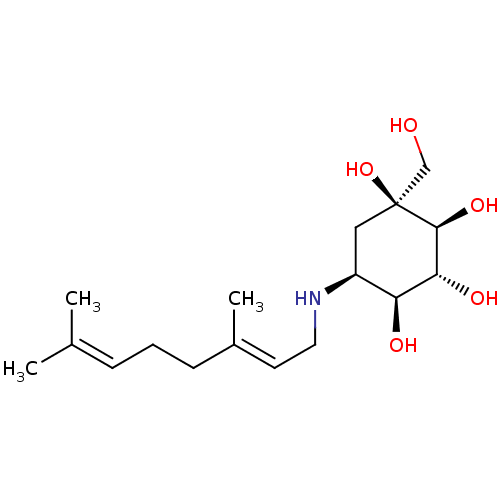 (5-(3,7-Dimethyl-octa-2,6-dienylamino)-1-hydroxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Homo sapiens (Human)) | BDBM50024124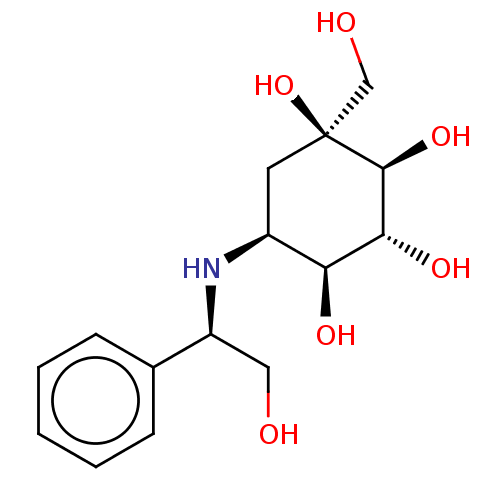 (1-Hydroxymethyl-5-(2-hydroxy-1-phenyl-ethylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase | J Med Chem 29: 1038-46 (1986) BindingDB Entry DOI: 10.7270/Q27P8ZZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 626 total ) | Next | Last >> |