| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Melanocortin receptor 4 |
|---|
| Ligand | BDBM50033134 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_106490 (CHEMBL716182) |
|---|
| Kd | 0.501000±n/a nM |
|---|
| Citation |  Hruby, VJ; Lu, D; Sharma, SD; Castrucci, AL; Kesterson, RA; al-Obeidi, FA; Hadley, ME; Cone, RD Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem38:3454-61 (1995) [PubMed] Hruby, VJ; Lu, D; Sharma, SD; Castrucci, AL; Kesterson, RA; al-Obeidi, FA; Hadley, ME; Cone, RD Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem38:3454-61 (1995) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Melanocortin receptor 4 |
|---|
| Name: | Melanocortin receptor 4 |
|---|
| Synonyms: | MC4-R | MC4R | MC4R_HUMAN | Melanocortin MC4 | Melanocortin receptor 4 (MC-4) | Melanocortin receptor 4 (MC4-R) | Melanocortin receptor 4 (MC4R) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 36949.50 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P32245 |
|---|
| Residue: | 332 |
|---|
| Sequence: | MVNSTHRGMHTSLHLWNRSSYRLHSNASESLGKGYSDGGCYEQLFVSPEVFVTLGVISLL
ENILVIVAIAKNKNLHSPMYFFICSLAVADMLVSVSNGSETIVITLLNSTDTDAQSFTVN
IDNVIDSVICSSLLASICSLLSIAVDRYFTIFYALQYHNIMTVKRVGIIISCIWAACTVS
GILFIIYSDSSAVIICLITMFFTMLALMASLYVHMFLMARLHIKRIAVLPGTGAIRQGAN
MKGAITLTILIGVFVVCWAPFFLHLIFYISCPQNPYCVCFMSHFNLYLILIMCNSIIDPL
IYALRSQELRKTFKEIICCYPLGGLCDLSSRY
|
|
|
|---|
| BDBM50033134 |
|---|
| n/a |
|---|
| Name | BDBM50033134 |
|---|
| Synonyms: | (3R,6S,9R,12S,15S,23R)-15-((S)-2-Acetylamino-hexanoylamino)-6-(3-guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)-3-(1H-indol-3-ylmethyl)-9-naphthalen-2-ylmethyl-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18hexaaza-cyclotricosane-23-carboxylic acid amide | 15-(2-Acetylamino-hexanoylamino)-6-(3-guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)-3-(1H-indol-3-ylmethyl)-9-naphthalen-2-ylmethyl-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18hexaaza-cyclotricosane-23-carboxylic acid | CHEMBL266417 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C54H71N15O9 |
|---|
| Mol. Mass. | 1074.2366 |
|---|
| SMILES | CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |
|---|
| Structure | 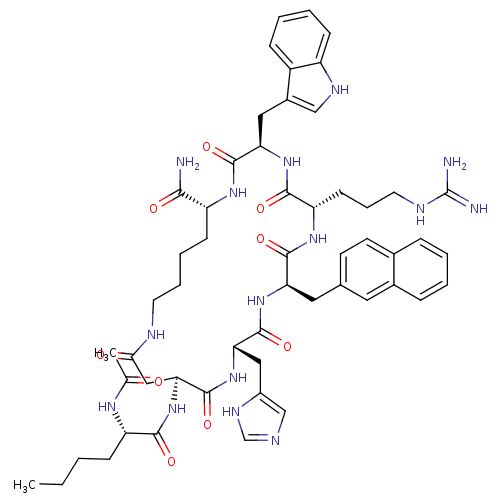
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hruby, VJ; Lu, D; Sharma, SD; Castrucci, AL; Kesterson, RA; al-Obeidi, FA; Hadley, ME; Cone, RD Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem38:3454-61 (1995) [PubMed]
Hruby, VJ; Lu, D; Sharma, SD; Castrucci, AL; Kesterson, RA; al-Obeidi, FA; Hadley, ME; Cone, RD Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem38:3454-61 (1995) [PubMed]