 Found 15417 hits with Last Name = 'lu' and Initial = 'd'
Found 15417 hits with Last Name = 'lu' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81811
(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50220746
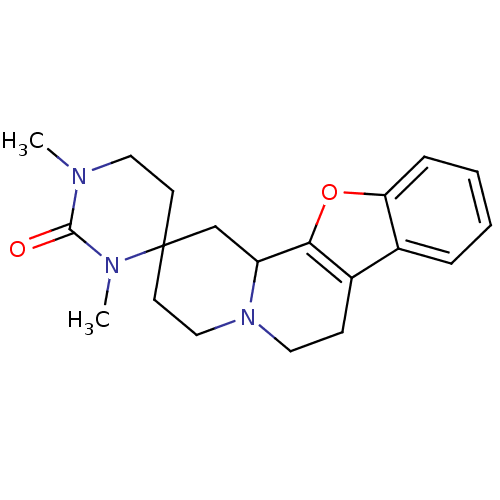
(CHEMBL292189)Show SMILES Cc1[nH]c(=O)n(CCCN2CCN(CC2)c2ccccc2OCC(F)(F)F)c(=O)c1C Show InChI InChI=1S/C21H27F3N4O3/c1-15-16(2)25-20(30)28(19(15)29)9-5-8-26-10-12-27(13-11-26)17-6-3-4-7-18(17)31-14-21(22,23)24/h3-4,6-7H,5,8-14H2,1-2H3,(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor |
Bioorg Med Chem Lett 13: 1873-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S46V50 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(OPOSSUM) | BDBM50013515

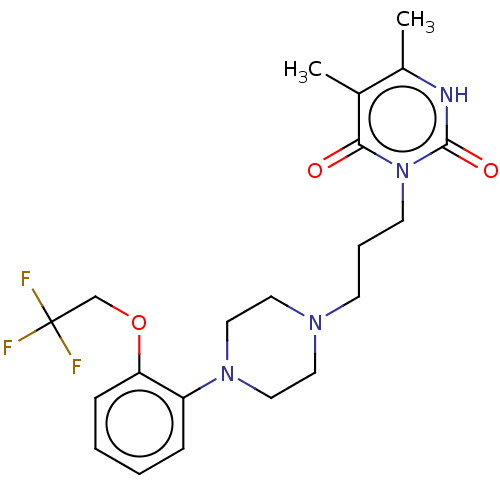
((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 244: 571-8 (1988)
BindingDB Entry DOI: 10.7270/Q25H7DS8 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 244: 571-8 (1988)
BindingDB Entry DOI: 10.7270/Q25H7DS8 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50080514
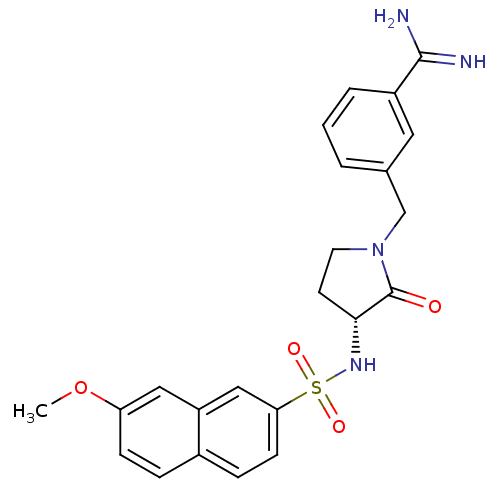
(3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H24N4O4S/c1-31-19-7-5-16-6-8-20(13-18(16)12-19)32(29,30)26-21-9-10-27(23(21)28)14-15-3-2-4-17(11-15)22(24)25/h2-8,11-13,21,26H,9-10,14H2,1H3,(H3,24,25)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of human Coagulation factor Xa |
J Med Chem 42: 3557-71 (1999)
Article DOI: 10.1021/jm990040h
BindingDB Entry DOI: 10.7270/Q2M04640 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81806
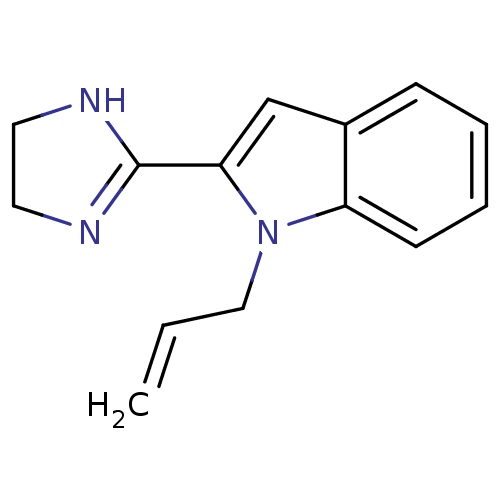
(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81806

(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81804
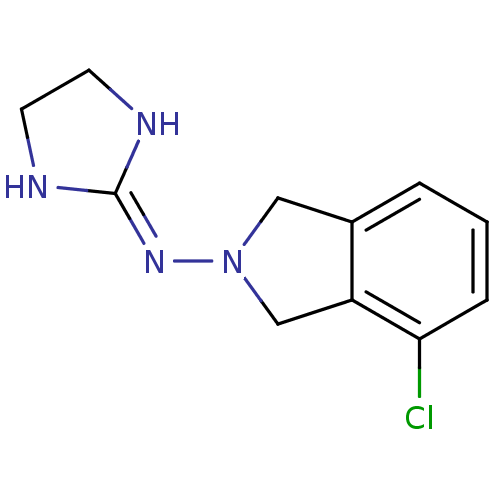
(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332270
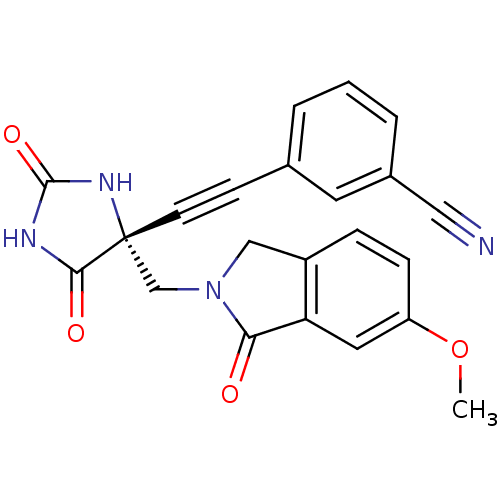
((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(c3)C#N)C(=O)c2c1 |r| Show InChI InChI=1S/C22H16N4O4/c1-30-17-6-5-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)8-7-14-3-2-4-15(9-14)11-23/h2-6,9-10H,12-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26526
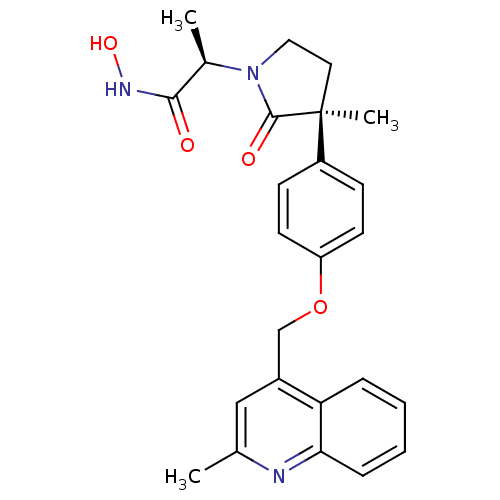
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50220747
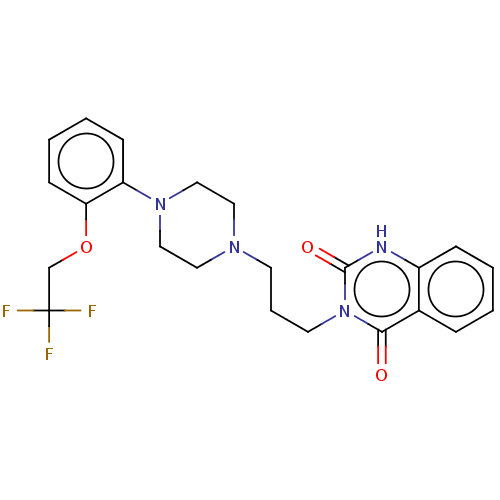
(CHEMBL56863)Show SMILES FC(F)(F)COc1ccccc1N1CCN(CCCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C23H25F3N4O3/c24-23(25,26)16-33-20-9-4-3-8-19(20)29-14-12-28(13-15-29)10-5-11-30-21(31)17-6-1-2-7-18(17)27-22(30)32/h1-4,6-9H,5,10-16H2,(H,27,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor |
Bioorg Med Chem Lett 13: 1873-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S46V50 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50444605
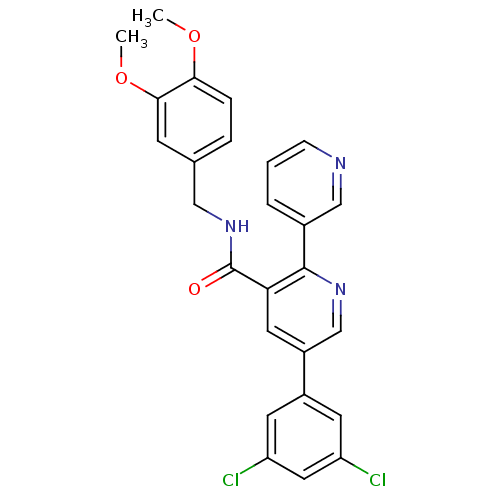
(CHEMBL3099899)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2cccnc2)-c2cc(Cl)cc(Cl)c2)cc1OC Show InChI InChI=1S/C26H21Cl2N3O3/c1-33-23-6-5-16(8-24(23)34-2)13-31-26(32)22-11-19(18-9-20(27)12-21(28)10-18)15-30-25(22)17-4-3-7-29-14-17/h3-12,14-15H,13H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 23: 6620-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.045
BindingDB Entry DOI: 10.7270/Q2WH2RFJ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332292
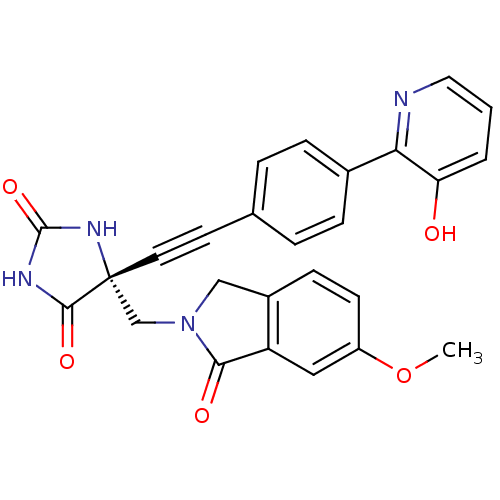
((R)-5-((4-(3-hydroxypyridin-2-yl)phenyl)ethynyl)-5...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H20N4O5/c1-35-19-9-8-18-14-30(23(32)20(18)13-19)15-26(24(33)28-25(34)29-26)11-10-16-4-6-17(7-5-16)22-21(31)3-2-12-27-22/h2-9,12-13,31H,14-15H2,1H3,(H2,28,29,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408198
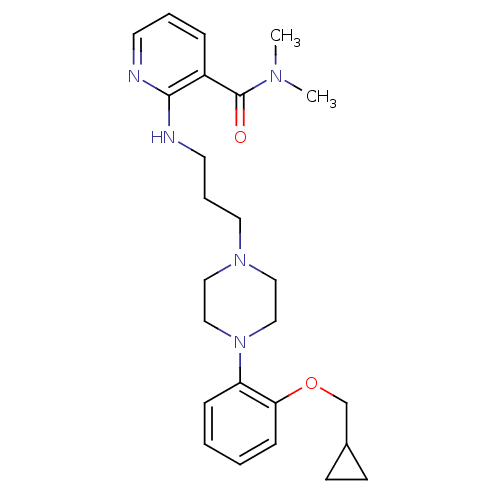
(CHEMBL91278)Show SMILES CN(C)C(=O)c1cccnc1NCCCN1CCN(CC1)c1ccccc1OCC1CC1 Show InChI InChI=1S/C25H35N5O2/c1-28(2)25(31)21-7-5-12-26-24(21)27-13-6-14-29-15-17-30(18-16-29)22-8-3-4-9-23(22)32-19-20-10-11-20/h3-5,7-9,12,20H,6,10-11,13-19H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81804

(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50474150
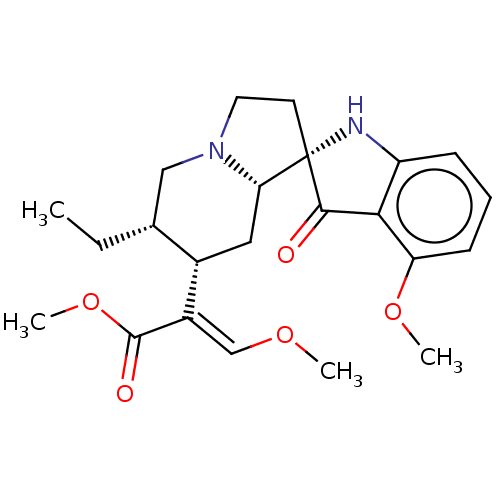
(CHEMBL58362)Show SMILES [H][C@@]12C[C@]([H])(C(=C/OC)\C(=O)OC)[C@]([H])(CC)CN1CC[C@]21Nc2cccc(OC)c2C1=O Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(19(25)11-15(14)16(13-28-2)22(27)30-4)21(26)20-17(24-23)7-6-8-18(20)29-3/h6-8,13-15,19,24H,5,9-12H2,1-4H3/b16-13+/t14-,15+,19+,23+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University
Curated by ChEMBL
| Assay Description
Binding affinity using guinea pig brain membrane preparations, towards Opioid receptor mu 1 using [3H]- DAMGO as radioligand |
J Med Chem 45: 1949-56 (2002)
Article DOI: 10.1021/jm010576e
BindingDB Entry DOI: 10.7270/Q2WS8X0T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM50062858
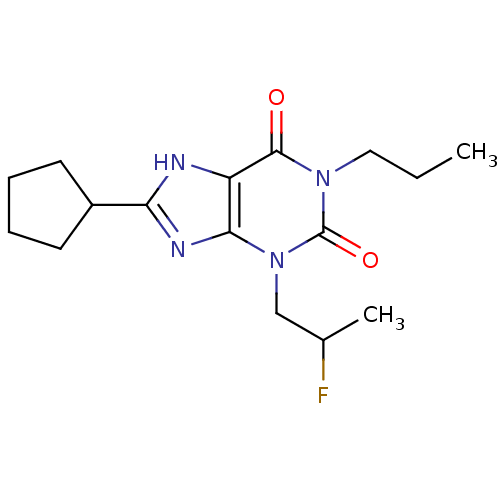
(8-Cyclopentyl-3-(2-fluoro-propyl)-1-propyl-3,7-dih...)Show InChI InChI=1S/C16H23FN4O2/c1-3-8-20-15(22)12-14(21(16(20)23)9-10(2)17)19-13(18-12)11-6-4-5-7-11/h10-11H,3-9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CPX from Adenosine A1 receptor of bovine brain cerebral cortex membranes |
J Med Chem 41: 555-63 (1998)
Article DOI: 10.1021/jm9705465
BindingDB Entry DOI: 10.7270/Q2RX9B60 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Binding affinity for opioid receptor like type, human Opioid receptor like 1 expressed in membrane homogenates of COS-1 or CHO cells |
J Med Chem 45: 5353-7 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1ST9 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325003
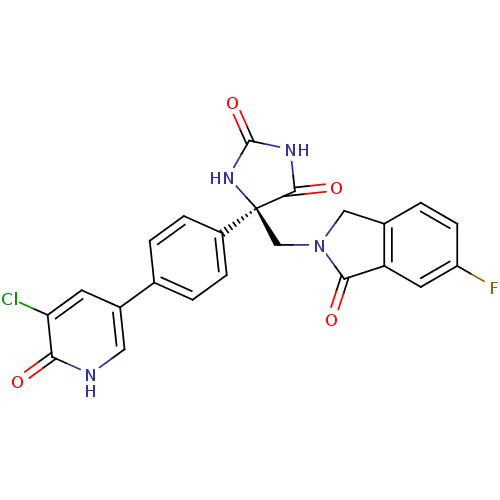
((R)-5-(4-(5-chloro-6-oxo-1,6-dihydropyridin-3-yl)p...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3c[nH]c(=O)c(Cl)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H16ClFN4O4/c24-18-7-14(9-26-19(18)30)12-1-4-15(5-2-12)23(21(32)27-22(33)28-23)11-29-10-13-3-6-16(25)8-17(13)20(29)31/h1-9H,10-11H2,(H,26,30)(H2,27,28,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM102669
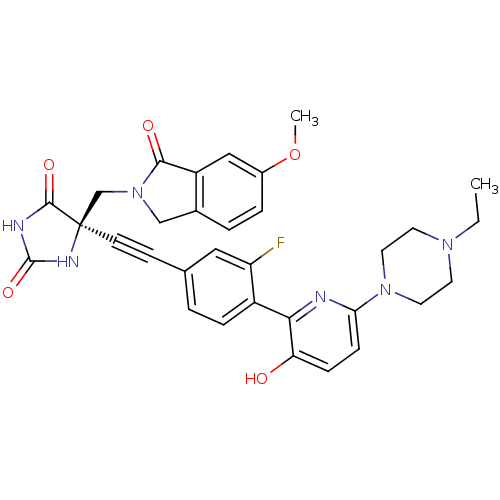
(CHEMBL1288726 | US8541572, 976)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1F)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H31FN6O5/c1-3-37-12-14-38(15-13-37)27-9-8-26(40)28(34-27)23-7-4-20(16-25(23)33)10-11-32(30(42)35-31(43)36-32)19-39-18-21-5-6-22(44-2)17-24(21)29(39)41/h4-9,16-17,40H,3,12-15,18-19H2,1-2H3,(H2,35,36,42,43)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 1362-7 (1994)
BindingDB Entry DOI: 10.7270/Q24X569K |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
Mol Pharmacol 42: 1-5 (1992)
BindingDB Entry DOI: 10.7270/Q2WH2NGR |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50302273
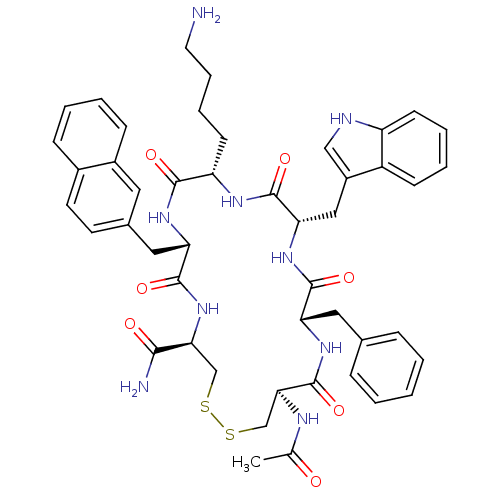
((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...)Show SMILES CC(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C47H55N9O7S2/c1-28(57)51-41-27-65-64-26-40(42(49)58)56-45(61)38(23-30-18-19-31-13-5-6-14-32(31)21-30)53-43(59)36(17-9-10-20-48)52-46(62)39(24-33-25-50-35-16-8-7-15-34(33)35)55-44(60)37(54-47(41)63)22-29-11-3-2-4-12-29/h2-8,11-16,18-19,21,25,36-41,50H,9-10,17,20,22-24,26-27,48H2,1H3,(H2,49,58)(H,51,57)(H,52,62)(H,53,59)(H,54,63)(H,55,60)(H,56,61)/t36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assay |
J Med Chem 52: 7432-45 (2009)
Article DOI: 10.1021/jm900683d
BindingDB Entry DOI: 10.7270/Q2S75GD7 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50408201
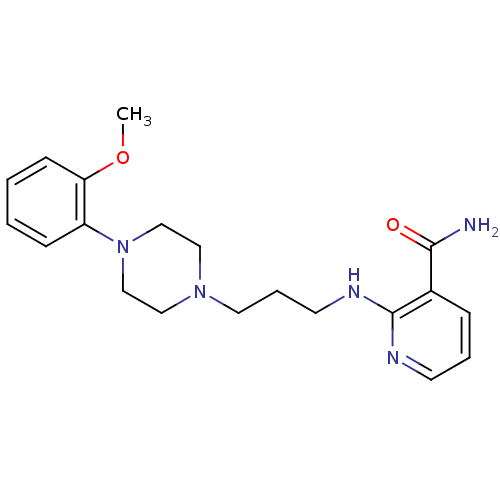
(CHEMBL88512)Show InChI InChI=1S/C20H27N5O2/c1-27-18-8-3-2-7-17(18)25-14-12-24(13-15-25)11-5-10-23-20-16(19(21)26)6-4-9-22-20/h2-4,6-9H,5,10-15H2,1H3,(H2,21,26)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(CALF) | BDBM50060964
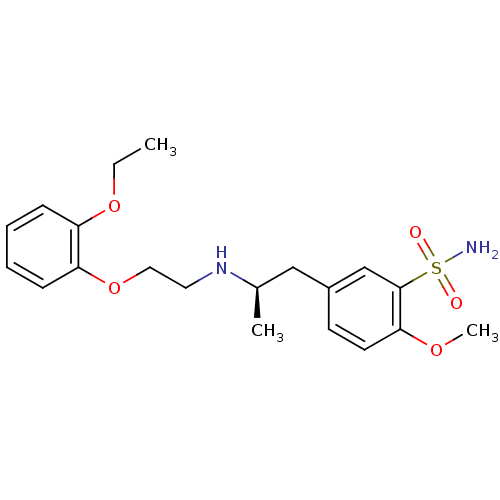
((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...)Show SMILES CCOc1ccccc1OCCN[C@H](C)Cc1ccc(OC)c(c1)S(N)(=O)=O Show InChI InChI=1S/C20H28N2O5S/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor |
J Med Chem 40: 2674-87 (1997)
Checked by Author
Article DOI: 10.1021/jm970166j
BindingDB Entry DOI: 10.7270/Q2R78GD2 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
Mol Pharmacol 42: 1-5 (1992)
BindingDB Entry DOI: 10.7270/Q2WH2NGR |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332265

((R)-5-((2-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3F)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-15-7-6-14-11-25(18(26)16(14)10-15)12-21(19(27)23-20(28)24-21)9-8-13-4-2-3-5-17(13)22/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50052442
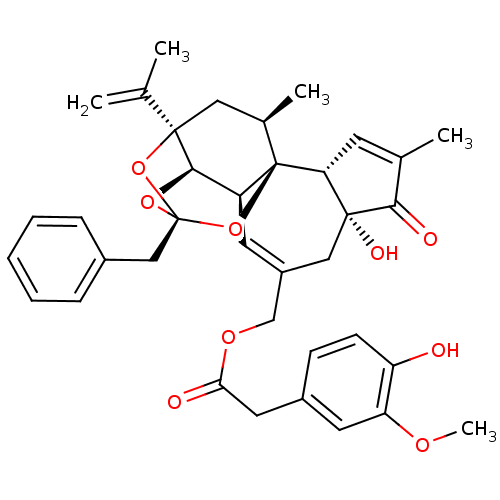
((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,THB:23:15:26.25.24:12| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35-,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin |
J Med Chem 46: 3116-26 (2003)
Article DOI: 10.1021/jm030089u
BindingDB Entry DOI: 10.7270/Q2SB4551 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50020192
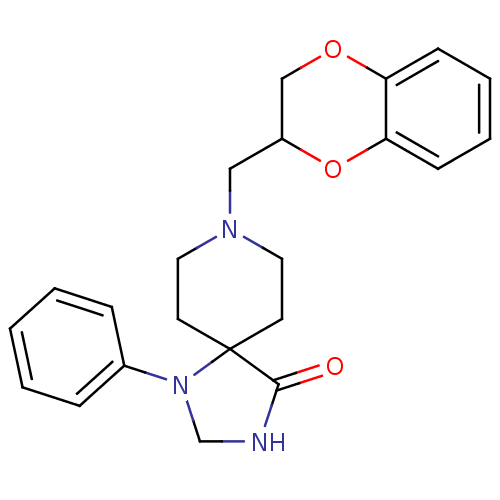
(8-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-1-phen...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC2COc3ccccc3O2)CC1 Show InChI InChI=1S/C22H25N3O3/c26-21-22(25(16-23-21)17-6-2-1-3-7-17)10-12-24(13-11-22)14-18-15-27-19-8-4-5-9-20(19)28-18/h1-9,18H,10-16H2,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
Mol Pharmacol 42: 1-5 (1992)
BindingDB Entry DOI: 10.7270/Q2WH2NGR |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM81804

(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81815
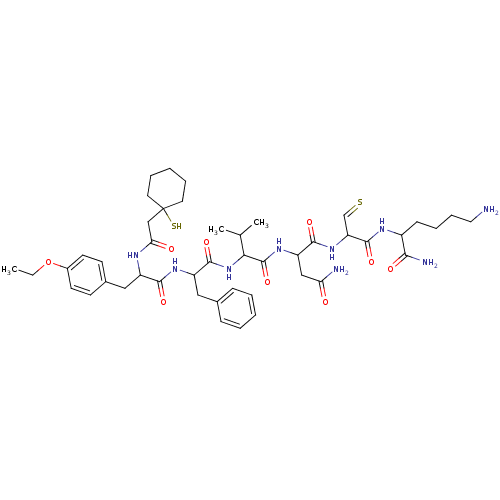
(CAS_196343 | L-654,284 | NSC_196343)Show SMILES CCOc1ccc(CC(NC(=O)CC2(S)CCCCC2)C(=O)NC(Cc2ccccc2)C(=O)NC(C(C)C)C(=O)NC(CC(N)=O)C(=O)NC(C=S)C(=O)NC(CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33(50-38(57)26-46(66)20-10-6-11-21-46)41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(27-65)44(62)51-32(40(49)58)15-9-12-22-47/h5,7-8,13-14,16-19,27-28,32-36,39,66H,4,6,9-12,15,20-26,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292694
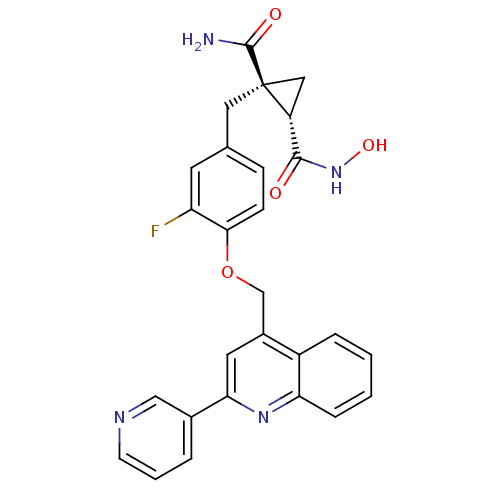
((1R,2S)-1-(3-fluoro-4-((2-(pyridin-3-yl)quinolin-4...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3cccnc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C27H23FN4O4/c28-21-10-16(12-27(26(29)34)13-20(27)25(33)32-35)7-8-24(21)36-15-18-11-23(17-4-3-9-30-14-17)31-22-6-2-1-5-19(18)22/h1-11,14,20,35H,12-13,15H2,(H2,29,34)(H,32,33)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332262
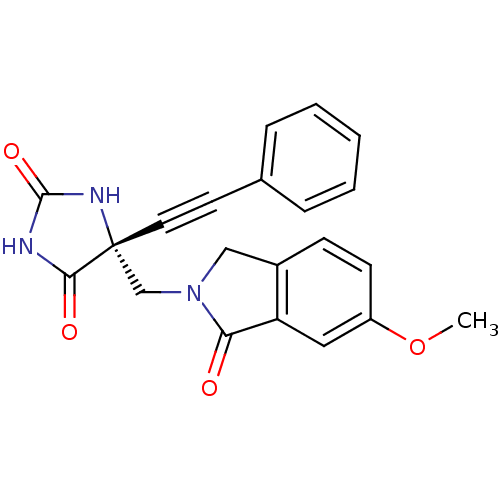
((R)-5-((6-methoxy-1-oxoisoindolin-2-yl)methyl)-5-(...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H17N3O4/c1-28-16-8-7-15-12-24(18(25)17(15)11-16)13-21(19(26)22-20(27)23-21)10-9-14-5-3-2-4-6-14/h2-8,11H,12-13H2,1H3,(H2,22,23,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50010483
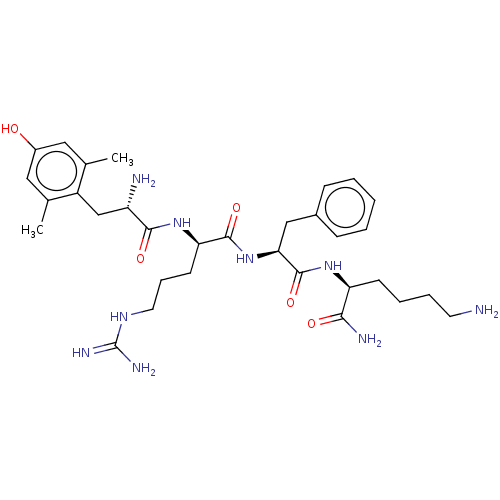
(CHEMBL2181202)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-type opioid receptor in rat brain membranes incubated for 2 hrs |
Bioorg Med Chem Lett 26: 3629-31 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.003
BindingDB Entry DOI: 10.7270/Q2ZW1NVQ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50499869

(CHEMBL3742140)Show SMILES OC(=O)C(F)(F)F.Nc1nc2CC[C@@H](Cc2s1)N(CCN1CCN(CC1)c1ccc2cc[nH]c2c1Cl)CC#N |r| Show InChI InChI=1S/C23H28ClN7S.C2HF3O2/c24-21-19(4-1-16-5-7-27-22(16)21)31-13-10-29(11-14-31)9-12-30(8-6-25)17-2-3-18-20(15-17)32-23(26)28-18;3-2(4,5)1(6)7/h1,4-5,7,17,27H,2-3,8-15H2,(H2,26,28);(H,6,7)/t17-;/m0./s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Spiroperidol from cloned rat dopamine D3 receptor expressed in HEK293 cells by liquid scintillation counting analysis |
J Med Chem 58: 9179-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01031
BindingDB Entry DOI: 10.7270/Q2N019HB |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292693
((1R,2S)-1-(3-fluoro-4-((2-(1-methyl-1H-pyrazol-3-y...)Show SMILES Cn1ccc(n1)-c1cc(COc2ccc(C[C@@]3(C[C@@H]3C(=O)NO)C(N)=O)cc2F)c2ccccc2n1 |r| Show InChI InChI=1S/C26H24FN5O4/c1-32-9-8-21(30-32)22-11-16(17-4-2-3-5-20(17)29-22)14-36-23-7-6-15(10-19(23)27)12-26(25(28)34)13-18(26)24(33)31-35/h2-11,18,35H,12-14H2,1H3,(H2,28,34)(H,31,33)/t18-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50292691

((1R,2S)-1-(3-fluoro-4-((2-(pyrrolidin-1-yl)quinoli...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)N3CCCC3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C26H27FN4O4/c27-20-11-16(13-26(25(28)33)14-19(26)24(32)30-34)7-8-22(20)35-15-17-12-23(31-9-3-4-10-31)29-21-6-2-1-5-18(17)21/h1-2,5-8,11-12,19,34H,3-4,9-10,13-15H2,(H2,28,33)(H,30,32)/t19-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TACE |
Bioorg Med Chem Lett 18: 5809-14 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.045
BindingDB Entry DOI: 10.7270/Q2N29WZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173
(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CPX from Adenosine A1 receptor of bovine brain cerebral cortex membranes |
J Med Chem 41: 555-63 (1998)
Article DOI: 10.1021/jm9705465
BindingDB Entry DOI: 10.7270/Q2RX9B60 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forschungszentrum Jülich GmbH
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CPX from Adenosine A1 receptor of bovine brain cerebral cortex membranes |
J Med Chem 41: 555-63 (1998)
Article DOI: 10.1021/jm9705465
BindingDB Entry DOI: 10.7270/Q2RX9B60 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50220750

(CHEMBL55695)Show SMILES Cc1c[nH]c(=O)n(CCCCN2CCN(CC2)c2ccc(F)cc2OCC(F)(F)F)c1=O Show InChI InChI=1S/C21H26F4N4O3/c1-15-13-26-20(31)29(19(15)30)7-3-2-6-27-8-10-28(11-9-27)17-5-4-16(22)12-18(17)32-14-21(23,24)25/h4-5,12-13H,2-3,6-11,14H2,1H3,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by ChEMBL
| Assay Description
Inhibition of [3H]prazosin binding to CHO-K1 whole cells expressing human cloned Alpha-1A adrenergic receptor |
Bioorg Med Chem Lett 13: 1873-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S46V50 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332289

((R)-5-((4-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C28H30N6O5/c1-3-32-12-14-33(15-13-32)24(31-38)20-6-4-19(5-7-20)10-11-28(26(36)29-27(37)30-28)18-34-17-21-8-9-22(39-2)16-23(21)25(34)35/h4-9,16,38H,3,12-15,17-18H2,1-2H3,(H2,29,30,36,37)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data