| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tissue-type plasminogen activator |
|---|
| Ligand | BDBM13280 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_208075 (CHEMBL813534) |
|---|
| Ki | >10000±n/a nM |
|---|
| Citation |  Ewing, WR; Becker, MR; Manetta, VE; Davis, RS; Pauls, HW; Mason, H; Choi-Sledeski, YM; Green, D; Cha, D; Spada, AP; Cheney, DL; Mason, JS; Maignan, S; Guilloteau, JP; Brown, K; Colussi, D; Bentley, R; Bostwick, J; Kasiewski, CJ; Morgan, SR; Leadley, RJ; Dunwiddie, CT; Perrone, MH; Chu, V Design and structure-activity relationships of potent and selective inhibitors of blood coagulation factor Xa. J Med Chem42:3557-71 (1999) [PubMed] Article Ewing, WR; Becker, MR; Manetta, VE; Davis, RS; Pauls, HW; Mason, H; Choi-Sledeski, YM; Green, D; Cha, D; Spada, AP; Cheney, DL; Mason, JS; Maignan, S; Guilloteau, JP; Brown, K; Colussi, D; Bentley, R; Bostwick, J; Kasiewski, CJ; Morgan, SR; Leadley, RJ; Dunwiddie, CT; Perrone, MH; Chu, V Design and structure-activity relationships of potent and selective inhibitors of blood coagulation factor Xa. J Med Chem42:3557-71 (1999) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tissue-type plasminogen activator |
|---|
| Name: | Tissue-type plasminogen activator |
|---|
| Synonyms: | Alteplase | PLAT | Reteplase | TPA_HUMAN | Thrombin receptor protein | Tissue-type plasminogen activator | Tissue-type plasminogen activator (tPA) | Tissue-type plasminogen activator precursor | t-PA | t-Plasminogen Activator (tPA) | t-plasminogen activator |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 62931.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 562 |
|---|
| Sequence: | MDAMKRGLCCVLLLCGAVFVSPSQEIHARFRRGARSYQVICRDEKTQMIYQQHQSWLRPV
LRSNRVEYCWCNSGRAQCHSVPVKSCSEPRCFNGGTCQQALYFSDFVCQCPEGFAGKCCE
IDTRATCYEDQGISYRGTWSTAESGAECTNWNSSALAQKPYSGRRPDAIRLGLGNHNYCR
NPDRDSKPWCYVFKAGKYSSEFCSTPACSEGNSDCYFGNGSAYRGTHSLTESGASCLPWN
SMILIGKVYTAQNPSAQALGLGKHNYCRNPDGDAKPWCHVLKNRRLTWEYCDVPSCSTCG
LRQYSQPQFRIKGGLFADIASHPWQAAIFAKHRRSPGERFLCGGILISSCWILSAAHCFQ
ERFPPHHLTVILGRTYRVVPGEEEQKFEVEKYIVHKEFDDDTYDNDIALLQLKSDSSRCA
QESSVVRTVCLPPADLQLPDWTECELSGYGKHEALSPFYSERLKEAHVRLYPSSRCTSQH
LLNRTVTDNMLCAGDTRSGGPQANLHDACQGDSGGPLVCLNDGRMTLVGIISWGLGCGQK
DVPGVYTKVTNYLDWIRDNMRP
|
|
|
|---|
| BDBM13280 |
|---|
| n/a |
|---|
| Name | BDBM13280 |
|---|
| Synonyms: | 3-{[(3S)-3-[(7-methoxynaphthalene-2-)sulfonamido]-2-oxopyrrolidin-1-yl]methyl}benzene-1-carboximidamide | CHEMBL327600 | Sulfonamidopyrrolidinone 3a |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H24N4O4S |
|---|
| Mol. Mass. | 452.526 |
|---|
| SMILES | COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O |r| |
|---|
| Structure | 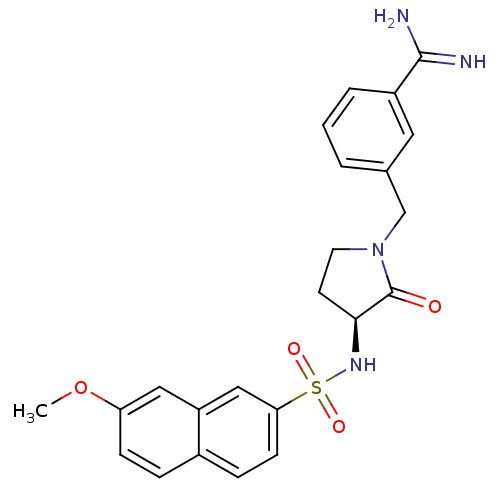
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ewing, WR; Becker, MR; Manetta, VE; Davis, RS; Pauls, HW; Mason, H; Choi-Sledeski, YM; Green, D; Cha, D; Spada, AP; Cheney, DL; Mason, JS; Maignan, S; Guilloteau, JP; Brown, K; Colussi, D; Bentley, R; Bostwick, J; Kasiewski, CJ; Morgan, SR; Leadley, RJ; Dunwiddie, CT; Perrone, MH; Chu, V Design and structure-activity relationships of potent and selective inhibitors of blood coagulation factor Xa. J Med Chem42:3557-71 (1999) [PubMed] Article
Ewing, WR; Becker, MR; Manetta, VE; Davis, RS; Pauls, HW; Mason, H; Choi-Sledeski, YM; Green, D; Cha, D; Spada, AP; Cheney, DL; Mason, JS; Maignan, S; Guilloteau, JP; Brown, K; Colussi, D; Bentley, R; Bostwick, J; Kasiewski, CJ; Morgan, SR; Leadley, RJ; Dunwiddie, CT; Perrone, MH; Chu, V Design and structure-activity relationships of potent and selective inhibitors of blood coagulation factor Xa. J Med Chem42:3557-71 (1999) [PubMed] Article