| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50092157 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_50175 (CHEMBL662233) |
|---|
| IC50 | 6400±n/a nM |
|---|
| Citation |  Low, CM; Black, JW; Broughton, HB; Buck, IM; Davies, JM; Dunstone, DJ; Hull, RA; Kalindjian, SB; McDonald, IM; Pether, MJ; Shankley, NP; Steel, KI Development of peptide 3D structure mimetics: rational design of novel peptoid cholecystokinin receptor antagonists. J Med Chem43:3505-17 (2000) [PubMed] Low, CM; Black, JW; Broughton, HB; Buck, IM; Davies, JM; Dunstone, DJ; Hull, RA; Kalindjian, SB; McDonald, IM; Pether, MJ; Shankley, NP; Steel, KI Development of peptide 3D structure mimetics: rational design of novel peptoid cholecystokinin receptor antagonists. J Med Chem43:3505-17 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49676.37 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin central 0 RAT::P30551 |
|---|
| Residue: | 444 |
|---|
| Sequence: | MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQI
LLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLK
DFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAAT
WCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVM
VVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQL
SSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAE
KHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEE
DGRTIRALLSRYSYSHMSTSAPPP
|
|
|
|---|
| BDBM50092157 |
|---|
| n/a |
|---|
| Name | BDBM50092157 |
|---|
| Synonyms: | (4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxylic acid (4-bromo-phenyl)-amide | (4R,5S)-N-(4-bromophenyl)-3-oxo-4,5-diphenylpyrazolidine-1-carboxamide | 3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxylic acid (4-bromo-phenyl)-amide(LY288512) | CHEMBL117281 | LY-288512 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H18BrN3O2 |
|---|
| Mol. Mass. | 436.301 |
|---|
| SMILES | Brc1ccc(NC(=O)N2NC(=O)[C@@H]([C@H]2c2ccccc2)c2ccccc2)cc1 |
|---|
| Structure | 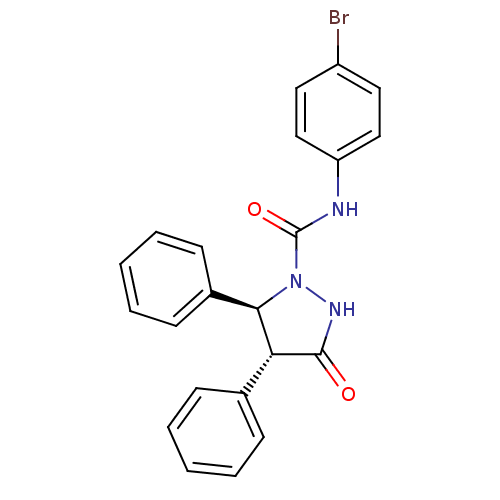
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Low, CM; Black, JW; Broughton, HB; Buck, IM; Davies, JM; Dunstone, DJ; Hull, RA; Kalindjian, SB; McDonald, IM; Pether, MJ; Shankley, NP; Steel, KI Development of peptide 3D structure mimetics: rational design of novel peptoid cholecystokinin receptor antagonists. J Med Chem43:3505-17 (2000) [PubMed]
Low, CM; Black, JW; Broughton, HB; Buck, IM; Davies, JM; Dunstone, DJ; Hull, RA; Kalindjian, SB; McDonald, IM; Pether, MJ; Shankley, NP; Steel, KI Development of peptide 3D structure mimetics: rational design of novel peptoid cholecystokinin receptor antagonists. J Med Chem43:3505-17 (2000) [PubMed]