

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536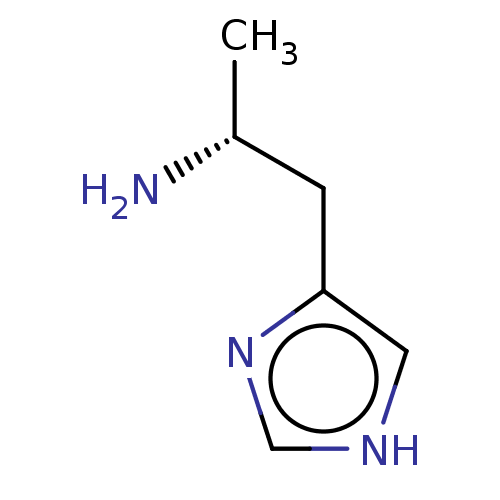 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411339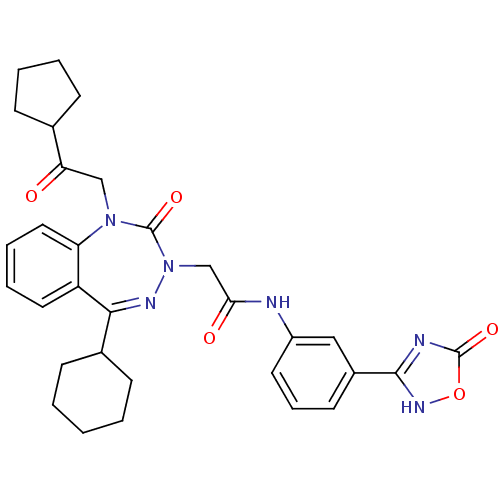 (CHEMBL227276) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50056102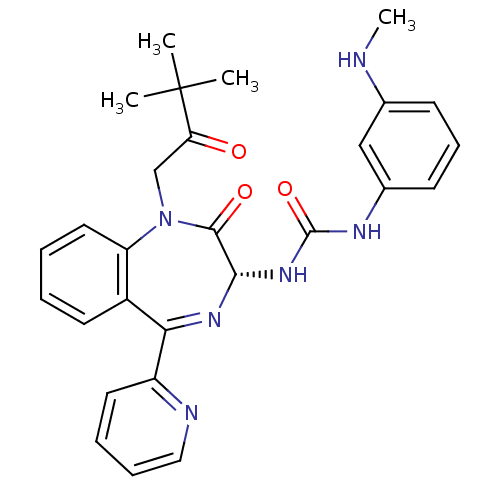 ((R)-1-(1-(3,3-dimethyl-2-oxobutyl)-2-oxo-5-(pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig ileum LMMP homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411341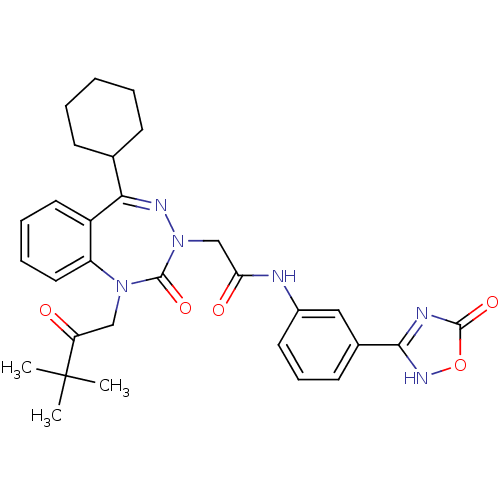 (CHEMBL226583) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002875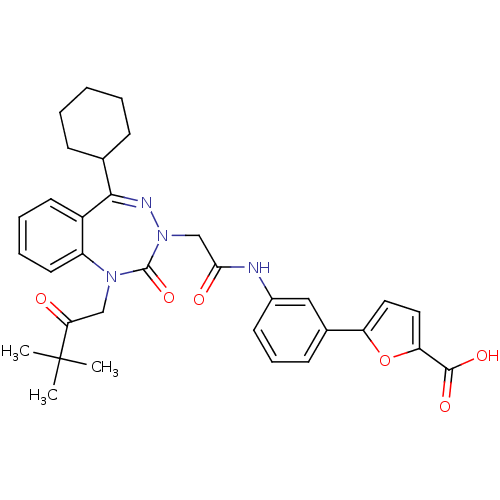 (CHEMBL388144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411340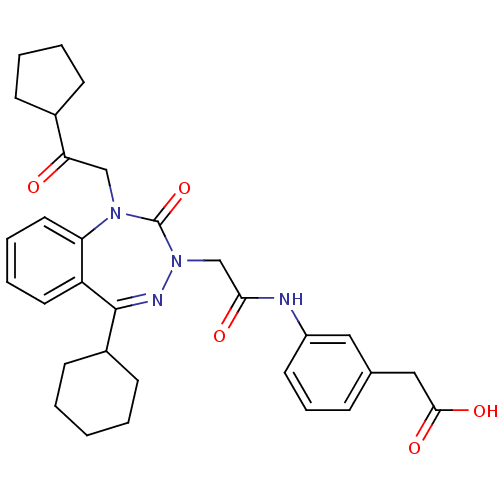 (CHEMBL387948) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411344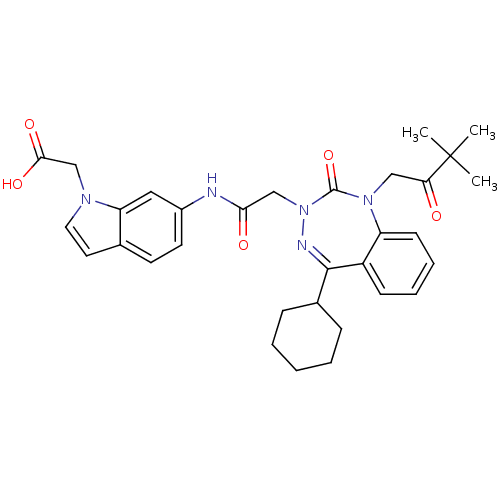 (CHEMBL389711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411342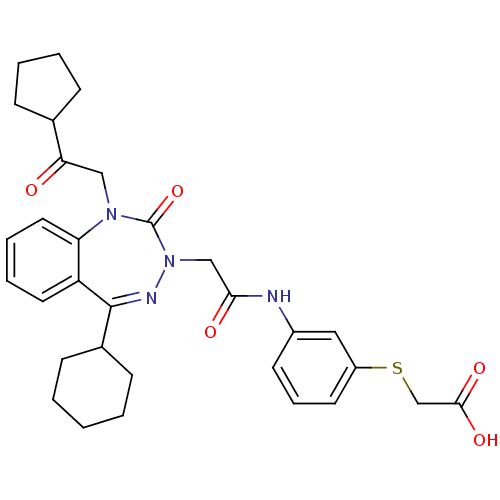 (CHEMBL227333) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411336 (CHEMBL227330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002880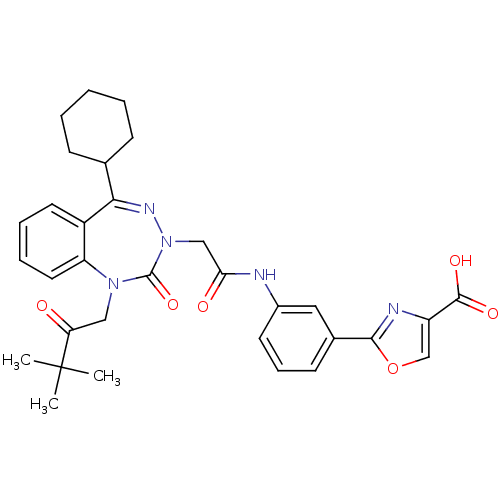 (CHEMBL437736) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411345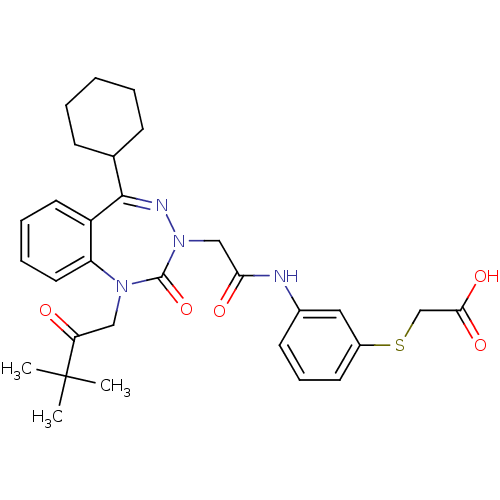 (CHEMBL389639) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002878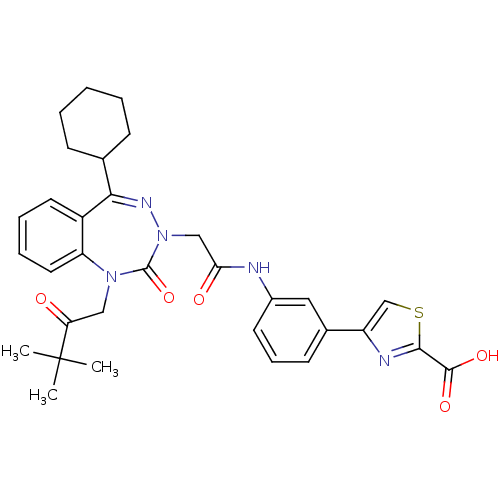 (CHEMBL227275) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002876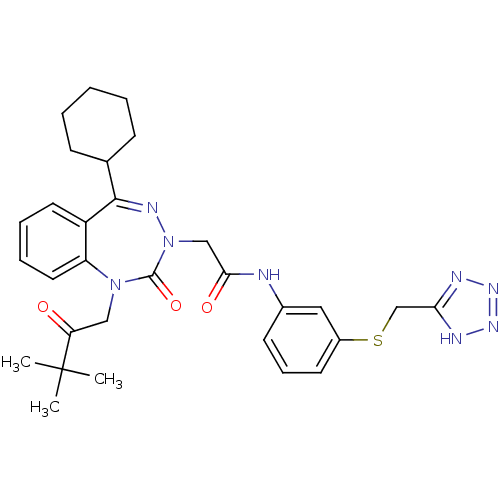 (CHEMBL435143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471066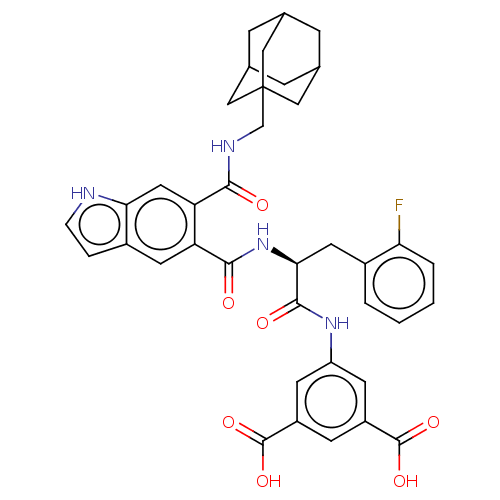 (CHEMBL415936) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002889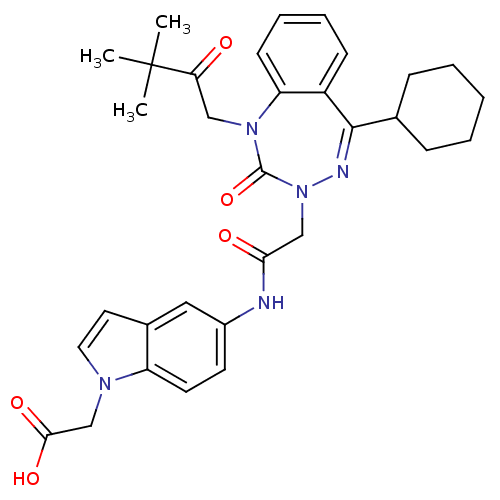 (CHEMBL438843) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002902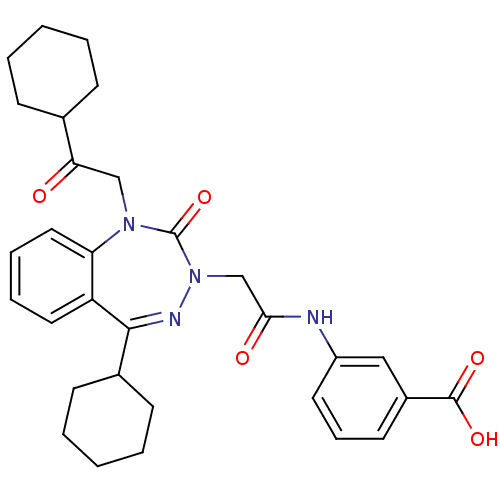 (CHEMBL226622) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002888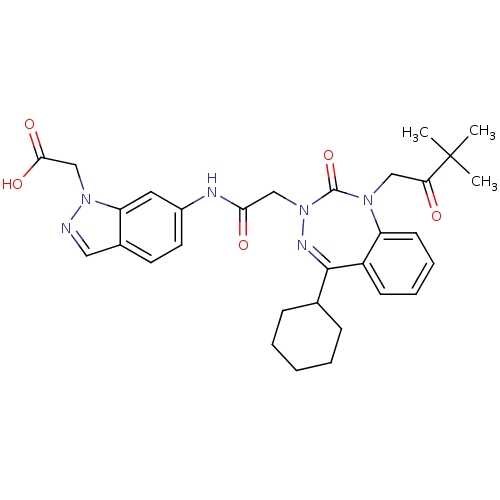 (CHEMBL389713) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002926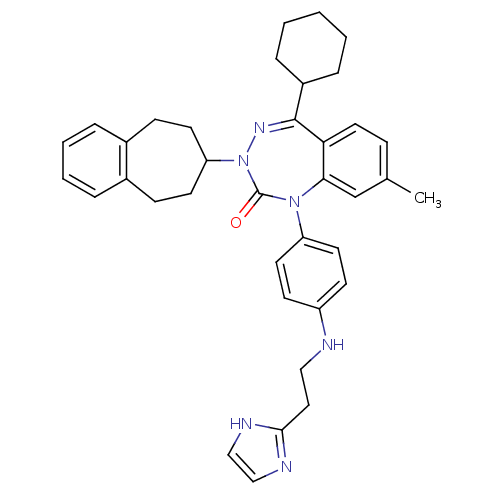 (CHEMBL244325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002872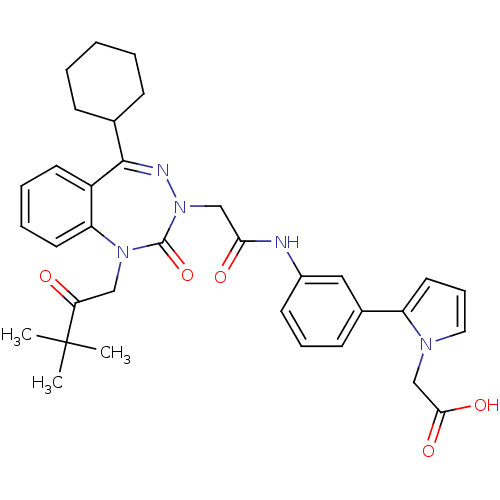 (CHEMBL437930) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215541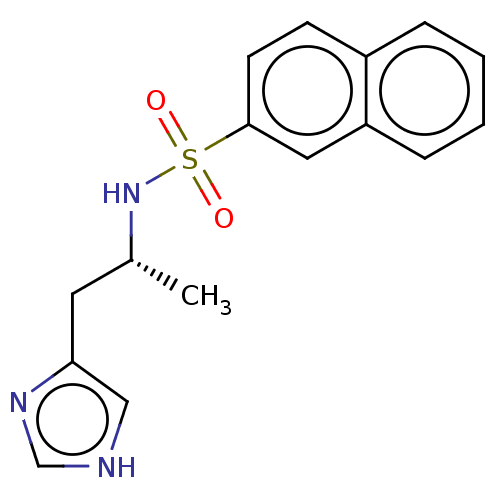 (CHEMBL299589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50213845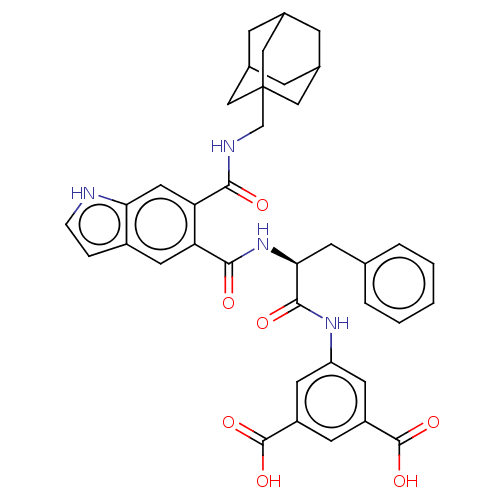 (CHEMBL14557) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411347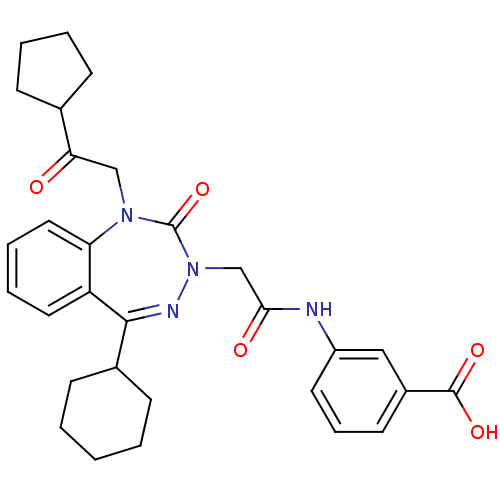 (CHEMBL226620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411348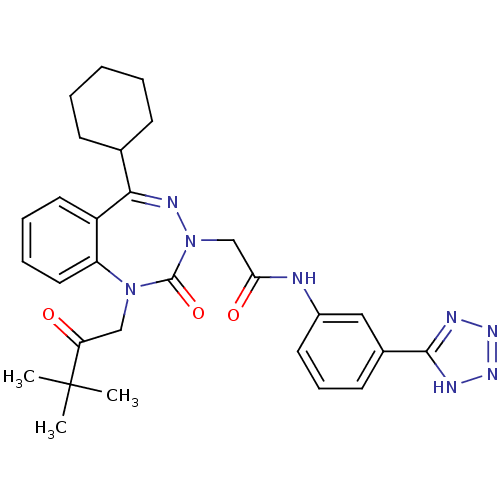 (CHEMBL226533) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002895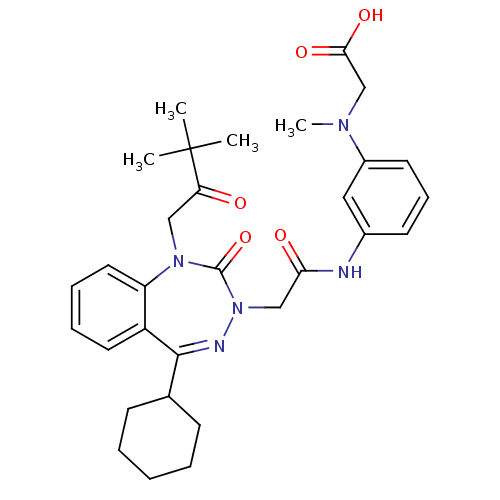 (CHEMBL226726) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50213845 (CHEMBL14557) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of binding of [125I]CCK-8S to Cholecystokinin type B receptor in mouse cerebral cortex homogenates | J Med Chem 43: 3518-29 (2000) Article DOI: 10.1021/jm000960w BindingDB Entry DOI: 10.7270/Q2KS6V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50213845 (CHEMBL14557) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM50398334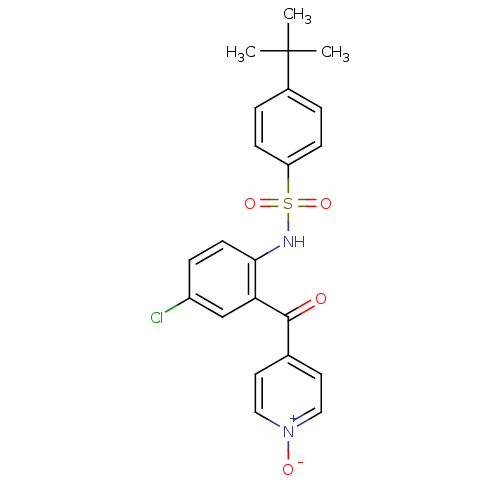 (CHEMBL2178578) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471067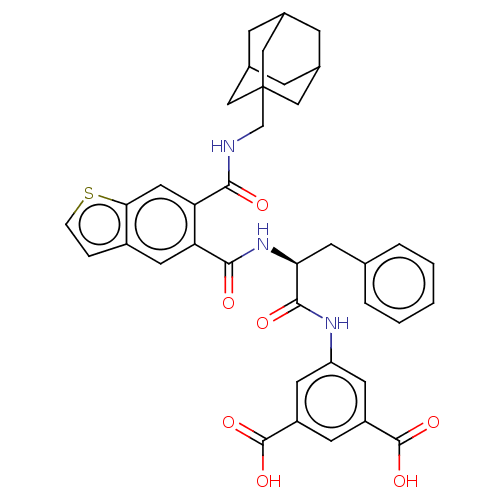 (CHEMBL298521) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471075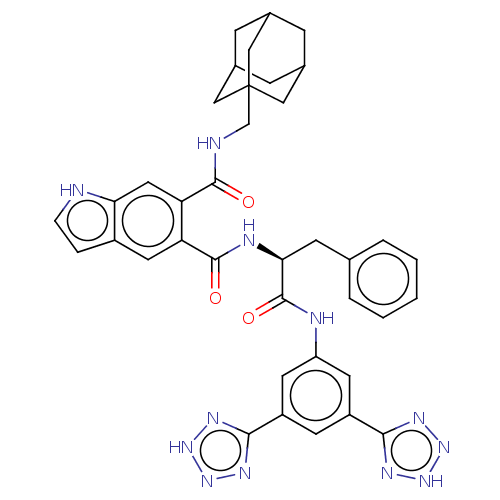 (CHEMBL299540) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002898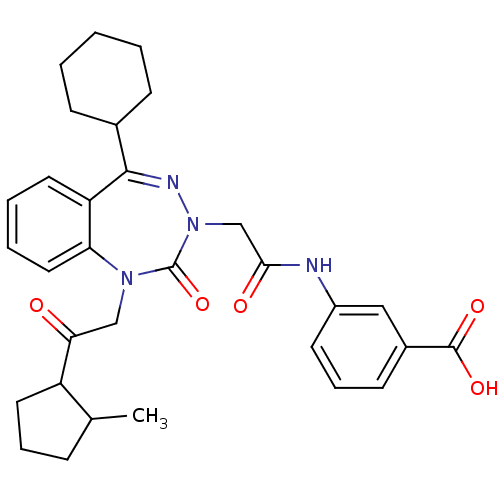 (CHEMBL388068) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002913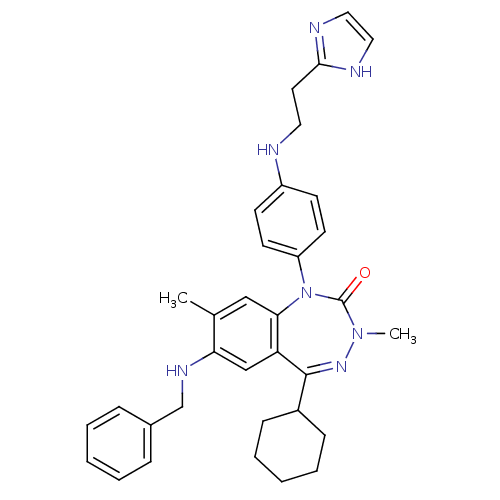 (CHEMBL389783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471065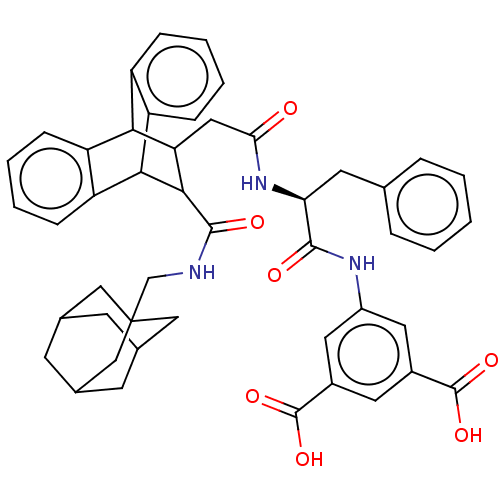 (CHEMBL296167) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes | J Med Chem 39: 1806-15 (1996) Article DOI: 10.1021/jm9508907 BindingDB Entry DOI: 10.7270/Q2571FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50470629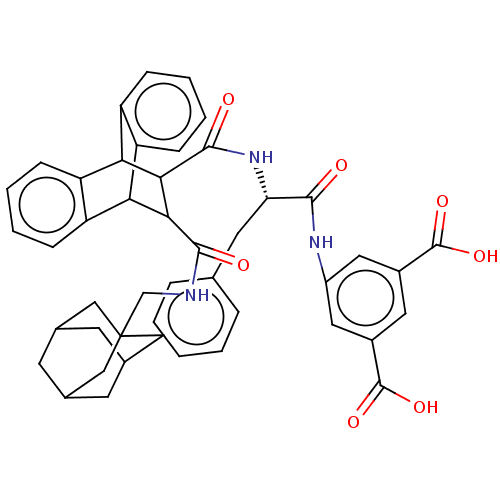 (CHEMBL342616) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002877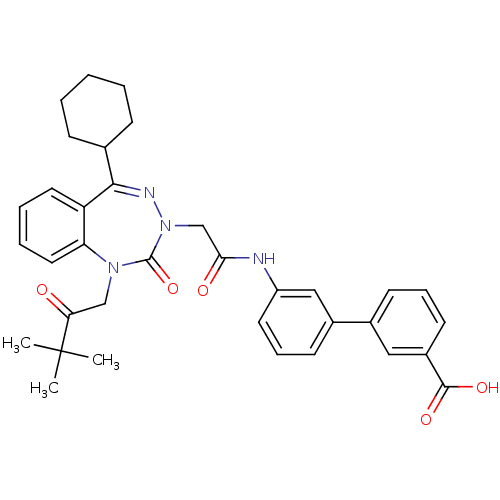 (CHEMBL388143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50002915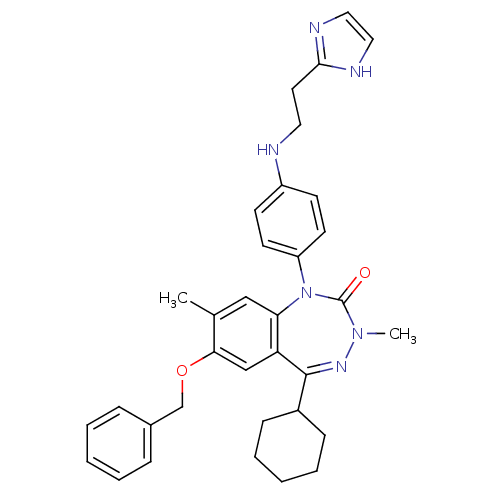 (CHEMBL389787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells | J Med Chem 50: 4789-92 (2007) Checked by Author Article DOI: 10.1021/jm0707626 BindingDB Entry DOI: 10.7270/Q2Z039C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411338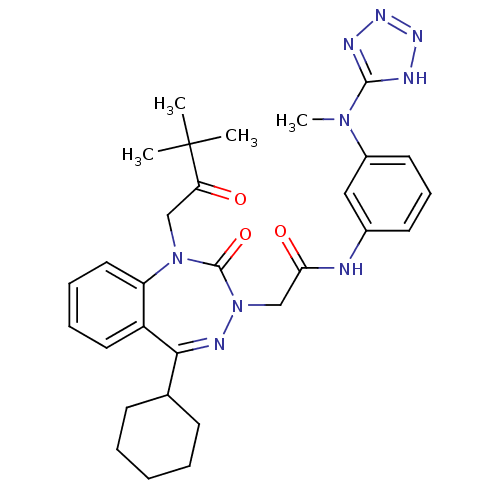 (CHEMBL227254) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411343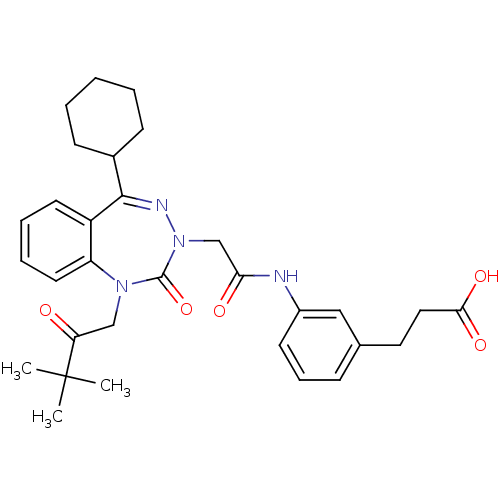 (CHEMBL226675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217235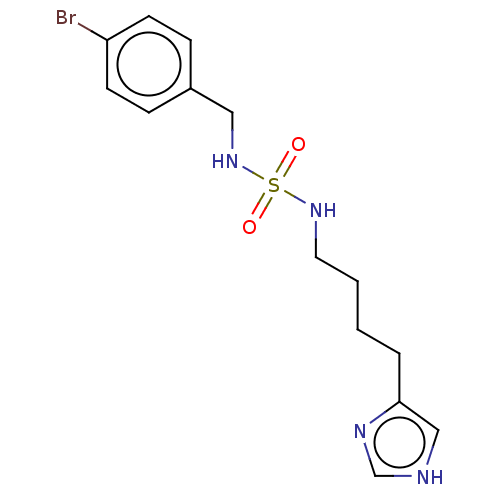 (CHEMBL104827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 9 (Homo sapiens (Human)) | BDBM393502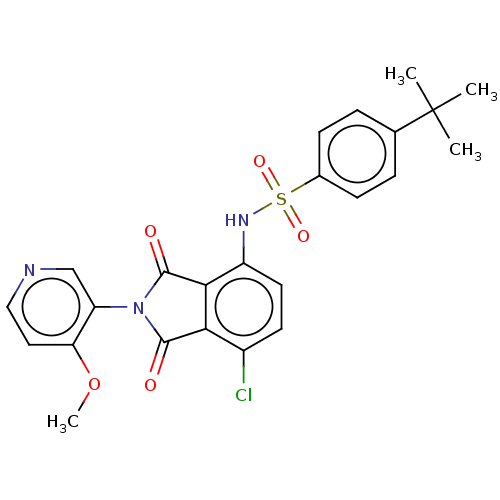 (US9969687, Compound 119) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Norgine Ltd Curated by ChEMBL | Assay Description Antagonist activity at CCR9 receptor in human MOLT4 cells assessed as inhibition of CCL25-induced increase in intracellular calcium level preincubate... | J Med Chem 59: 3098-111 (2016) Article DOI: 10.1021/acs.jmedchem.5b01840 BindingDB Entry DOI: 10.7270/Q28918WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411346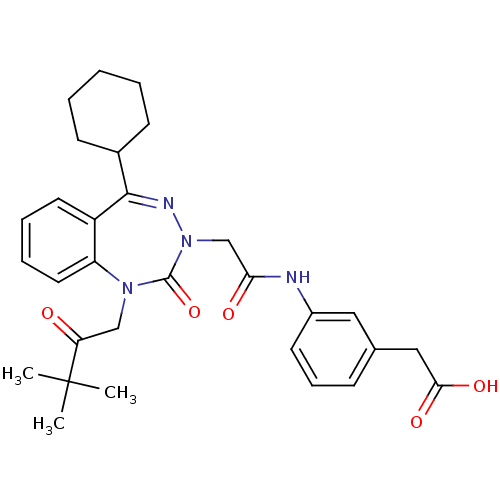 (CHEMBL226673) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217241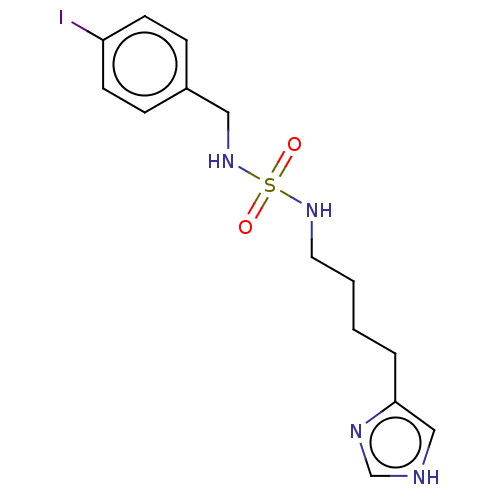 (CHEMBL104366) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50470612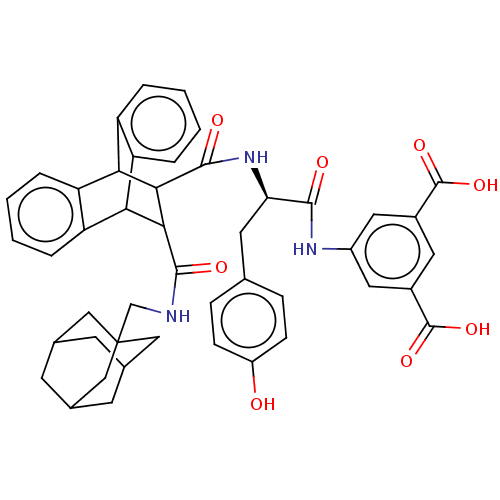 (CHEMBL436209) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates | J Med Chem 38: 4294-302 (1995) Article DOI: 10.1021/jm00021a019 BindingDB Entry DOI: 10.7270/Q2930WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50470623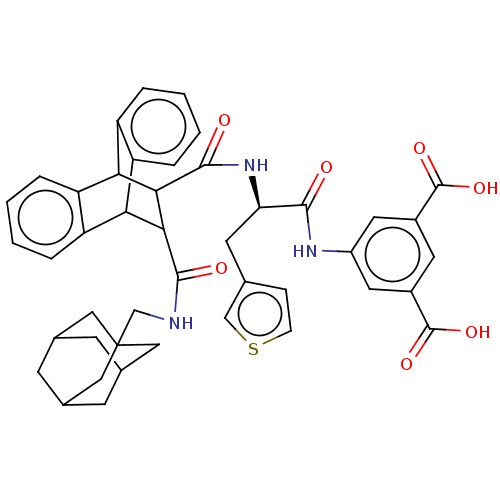 (CHEMBL140179) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates | J Med Chem 38: 4294-302 (1995) Article DOI: 10.1021/jm00021a019 BindingDB Entry DOI: 10.7270/Q2930WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Dog) | BDBM50411344 (CHEMBL389711) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosa | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217236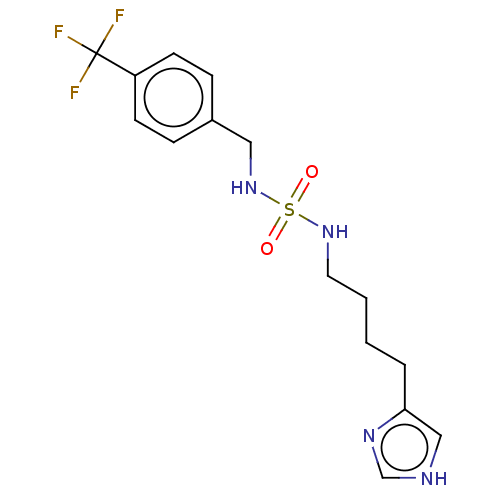 (CHEMBL105744) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50470617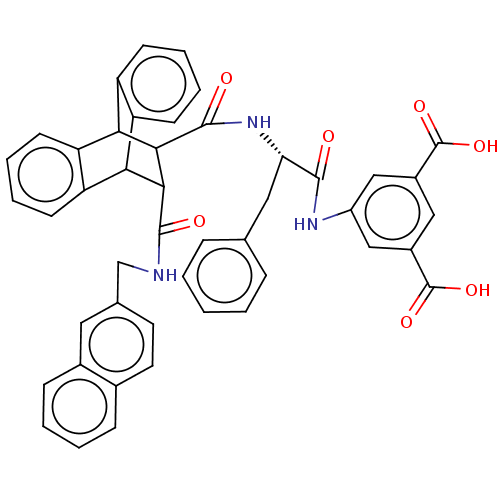 (CHEMBL137180) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates | J Med Chem 38: 4294-302 (1995) Article DOI: 10.1021/jm00021a019 BindingDB Entry DOI: 10.7270/Q2930WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002892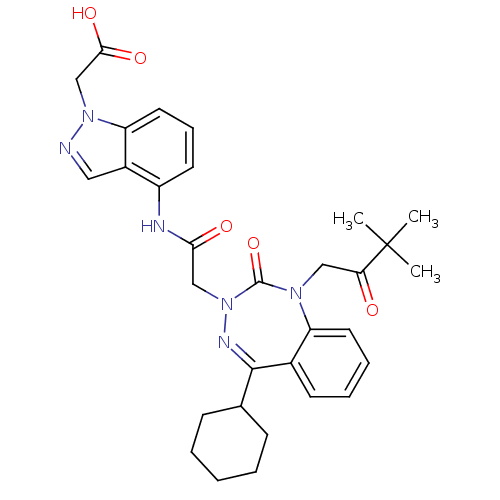 (CHEMBL1161950) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217238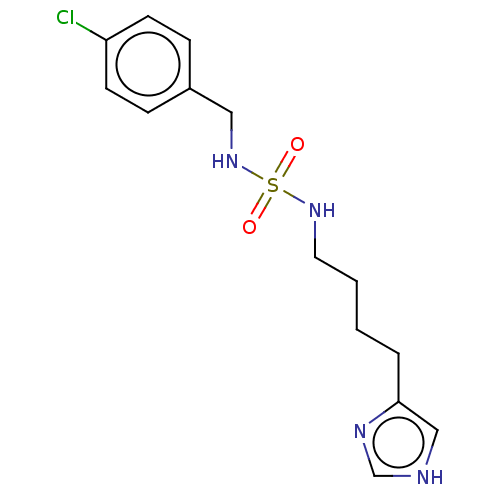 (CHEMBL107083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 777 total ) | Next | Last >> |