| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuromedin-K receptor |
|---|
| Ligand | BDBM50118099 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_209569 (CHEMBL811271) |
|---|
| Ki | 9.4±n/a nM |
|---|
| Citation |  Albert, JS; Aharony, D; Andisik, D; Barthlow, H; Bernstein, PR; Bialecki, RA; Dedinas, R; Dembofsky, BT; Hill, D; Kirkland, K; Koether, GM; Kosmider, BJ; Ohnmacht, C; Palmer, W; Potts, W; Rumsey, W; Shen, L; Shenvi, A; Sherwood, S; Warwick, PJ; Russell, K Design, synthesis, and SAR of tachykinin antagonists: modulation of balance in NK(1)/NK(2) receptor antagonist activity. J Med Chem45:3972-83 (2002) [PubMed] Albert, JS; Aharony, D; Andisik, D; Barthlow, H; Bernstein, PR; Bialecki, RA; Dedinas, R; Dembofsky, BT; Hill, D; Kirkland, K; Koether, GM; Kosmider, BJ; Ohnmacht, C; Palmer, W; Potts, W; Rumsey, W; Shen, L; Shenvi, A; Sherwood, S; Warwick, PJ; Russell, K Design, synthesis, and SAR of tachykinin antagonists: modulation of balance in NK(1)/NK(2) receptor antagonist activity. J Med Chem45:3972-83 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neuromedin-K receptor |
|---|
| Name: | Neuromedin-K receptor |
|---|
| Synonyms: | NK-3 receptor | NK-3R | NK3R | NK3R_HUMAN | NKR | Neurokinin 3 receptor | Neurokinin B receptor | Neurokinin-3 (NK-3) | Neuromedin-3 receptor (NK-3R) | Neuromedin-3 receptor (NK3) | Neuromedin-K receptor | Neuromedin-K receptor (NK-3 receptor) | Neuromedin-K receptor (NK3) | Neuromedin-K receptor(NK3R) | TAC3R | TACR3 | Tachykinin receptor 3 | Tachykinin receptor 3 (NK3) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52221.96 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29371 |
|---|
| Residue: | 465 |
|---|
| Sequence: | MATLPAAETWIDGGGGVGADAVNLTASLAAGAATGAVETGWLQLLDQAGNLSSSPSALGL
PVASPAPSQPWANLTNQFVQPSWRIALWSLAYGVVVAVAVLGNLIVIWIILAHKRMRTVT
NYFLVNLAFSDASMAAFNTLVNFIYALHSEWYFGANYCRFQNFFPITAVFASIYSMTAIA
VDRYMAIIDPLKPRLSATATKIVIGSIWILAFLLAFPQCLYSKTKVMPGRTLCFVQWPEG
PKQHFTYHIIVIILVYCFPLLIMGITYTIVGITLWGGEIPGDTCDKYHEQLKAKRKVVKM
MIIVVMTFAICWLPYHIYFILTAIYQQLNRWKYIQQVYLASFWLAMSSTMYNPIIYCCLN
KRFRAGFKRAFRWCPFIKVSSYDELELKTTRFHPNRQSSMYTVTRMESMTVVFDPNDADT
TRSSRKKRATPRDPSFNGCSRRNSKSASATSSFISSPYTSVDEYS
|
|
|
|---|
| BDBM50118099 |
|---|
| n/a |
|---|
| Name | BDBM50118099 |
|---|
| Synonyms: | 1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-methyl-amino]-3-(3,4-dichloro-phenyl)-butyl]-2-oxo-[1,4']bipiperidinyl-4'-carboxylic acid methylamide | CHEMBL339051 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C36H41Cl2N5O4 |
|---|
| Mol. Mass. | 678.648 |
|---|
| SMILES | CNC(=O)C1(CCN(CC[C@H](CN(C)C(=O)c2c(OC)c(cc3ccccc23)C#N)c2ccc(Cl)c(Cl)c2)CC1)N1CCCCC1=O |
|---|
| Structure | 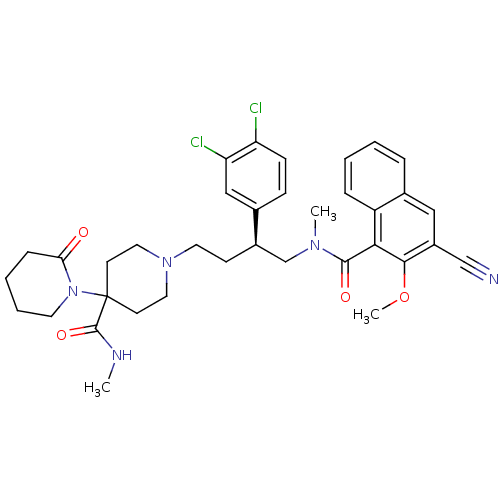
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Albert, JS; Aharony, D; Andisik, D; Barthlow, H; Bernstein, PR; Bialecki, RA; Dedinas, R; Dembofsky, BT; Hill, D; Kirkland, K; Koether, GM; Kosmider, BJ; Ohnmacht, C; Palmer, W; Potts, W; Rumsey, W; Shen, L; Shenvi, A; Sherwood, S; Warwick, PJ; Russell, K Design, synthesis, and SAR of tachykinin antagonists: modulation of balance in NK(1)/NK(2) receptor antagonist activity. J Med Chem45:3972-83 (2002) [PubMed]
Albert, JS; Aharony, D; Andisik, D; Barthlow, H; Bernstein, PR; Bialecki, RA; Dedinas, R; Dembofsky, BT; Hill, D; Kirkland, K; Koether, GM; Kosmider, BJ; Ohnmacht, C; Palmer, W; Potts, W; Rumsey, W; Shen, L; Shenvi, A; Sherwood, S; Warwick, PJ; Russell, K Design, synthesis, and SAR of tachykinin antagonists: modulation of balance in NK(1)/NK(2) receptor antagonist activity. J Med Chem45:3972-83 (2002) [PubMed]