| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone acetyltransferase KAT2B |
|---|
| Ligand | BDBM50247615 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_495684 (CHEMBL1006963) |
|---|
| IC50 | 3100±n/a nM |
|---|
| Citation |  Gorsuch, S; Bavetsias, V; Rowlands, MG; Aherne, GW; Workman, P; Jarman, M; McDonald, E Synthesis of isothiazol-3-one derivatives as inhibitors of histone acetyltransferases (HATs). Bioorg Med Chem17:467-74 (2009) [PubMed] Article Gorsuch, S; Bavetsias, V; Rowlands, MG; Aherne, GW; Workman, P; Jarman, M; McDonald, E Synthesis of isothiazol-3-one derivatives as inhibitors of histone acetyltransferases (HATs). Bioorg Med Chem17:467-74 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone acetyltransferase KAT2B |
|---|
| Name: | Histone acetyltransferase KAT2B |
|---|
| Synonyms: | Histone acetyltransferase KAT2A/KAT2B | Histone acetyltransferase PCAF | KAT2B | KAT2B_HUMAN | PCAF |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 93045.76 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1502838 |
|---|
| Residue: | 832 |
|---|
| Sequence: | MSEAGGAGPGGCGAGAGAGAGPGALPPQPAALPPAPPQGSPCAAAAGGSGACGPATAVAA
AGTAEGPGGGGSARIAVKKAQLRSAPRAKKLEKLGVYSACKAEESCKCNGWKNPNPSPTP
PRADLQQIIVSLTESCRSCSHALAAHVSHLENVSEEEMNRLLGIVLDVEYLFTCVHKEED
ADTKQVYFYLFKLLRKSILQRGKPVVEGSLEKKPPFEKPSIEQGVNNFVQYKFSHLPAKE
RQTIVELAKMFLNRINYWHLEAPSQRRLRSPNDDISGYKENYTRWLCYCNVPQFCDSLPR
YETTQVFGRTLLRSVFTVMRRQLLEQARQEKDKLPLEKRTLILTHFPKFLSMLEEEVYSQ
NSPIWDQDFLSASSRTSQLGIQTVINPPPVAGTISYNSTSSSLEQPNAGSSSPACKASSG
LEANPGEKRKMTDSHVLEEAKKPRVMGDIPMELINEVMSTITDPAAMLGPETNFLSAHSA
RDEAARLEERRGVIEFHVVGNSLNQKPNKKILMWLVGLQNVFSHQLPRMPKEYITRLVFD
PKHKTLALIKDGRVIGGICFRMFPSQGFTEIVFCAVTSNEQVKGYGTHLMNHLKEYHIKH
DILNFLTYADEYAIGYFKKQGFSKEIKIPKTKYVGYIKDYEGATLMGCELNPRIPYTEFS
VIIKKQKEIIKKLIERKQAQIRKVYPGLSCFKDGVRQIPIESIPGIRETGWKPSGKEKSK
EPRDPDQLYSTLKSILQQVKSHQSAWPFMEPVKRTEAPGYYEVIRFPMDLKTMSERLKNR
YYVSKKLFMADLQRVFTNCKEYNPPESEYYKCANILEKFFFSKIKEAGLIDK
|
|
|
|---|
| BDBM50247615 |
|---|
| n/a |
|---|
| Name | BDBM50247615 |
|---|
| Synonyms: | 2-(3,4,5-trifluorophenyl)isothiazol-3(2H)-one | CHEMBL489101 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C9H4F3NOS |
|---|
| Mol. Mass. | 231.194 |
|---|
| SMILES | Fc1cc(cc(F)c1F)-n1sccc1=O |
|---|
| Structure | 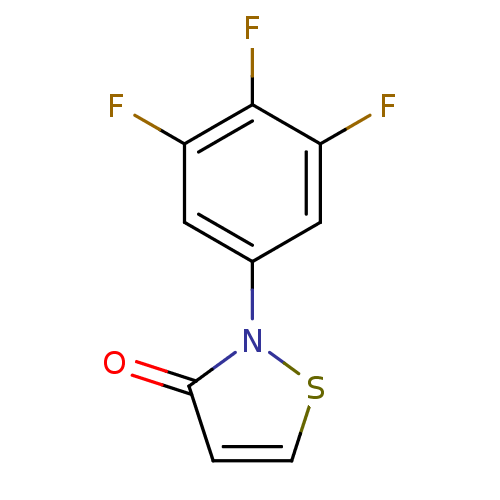
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gorsuch, S; Bavetsias, V; Rowlands, MG; Aherne, GW; Workman, P; Jarman, M; McDonald, E Synthesis of isothiazol-3-one derivatives as inhibitors of histone acetyltransferases (HATs). Bioorg Med Chem17:467-74 (2009) [PubMed] Article
Gorsuch, S; Bavetsias, V; Rowlands, MG; Aherne, GW; Workman, P; Jarman, M; McDonald, E Synthesis of isothiazol-3-one derivatives as inhibitors of histone acetyltransferases (HATs). Bioorg Med Chem17:467-74 (2009) [PubMed] Article