 Found 452 hits with Last Name = 'jarman' and Initial = 'm'
Found 452 hits with Last Name = 'jarman' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM50405683

(CHEMBL174909)Show InChI InChI=1S/C15H18N2O2/c1-2-3-8-17-13(18)12-9-15(12,14(17)19)10-4-6-11(16)7-5-10/h4-7,12H,2-3,8-9,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Apparent inhibition constant (Ki) for cytochrome P450 19A1 with androstenedione |
J Med Chem 31: 971-6 (1988)
BindingDB Entry DOI: 10.7270/Q2154J7X |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011767
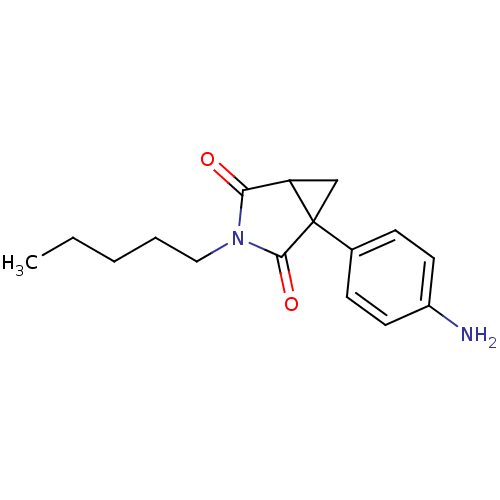
(1-(4-Amino-phenyl)-3-pentyl-3-aza-bicyclo[3.1.0]he...)Show InChI InChI=1S/C16H20N2O2/c1-2-3-4-9-18-14(19)13-10-16(13,15(18)20)11-5-7-12(17)8-6-11/h5-8,13H,2-4,9-10,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 androstenedione |
J Med Chem 31: 971-6 (1988)
BindingDB Entry DOI: 10.7270/Q2154J7X |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50405688
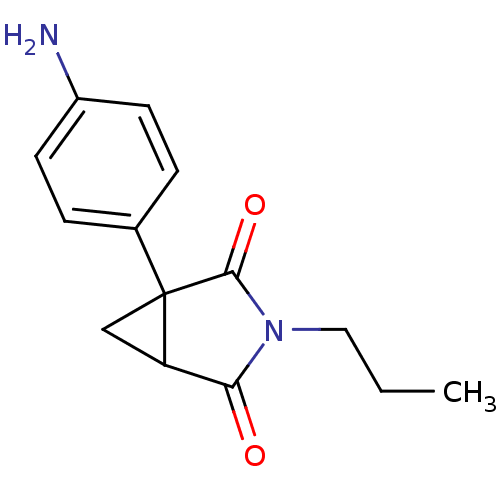
(CHEMBL174407)Show InChI InChI=1S/C14H16N2O2/c1-2-7-16-12(17)11-8-14(11,13(16)18)9-3-5-10(15)6-4-9/h3-6,11H,2,7-8,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 androstenedione |
J Med Chem 31: 971-6 (1988)
BindingDB Entry DOI: 10.7270/Q2154J7X |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024544
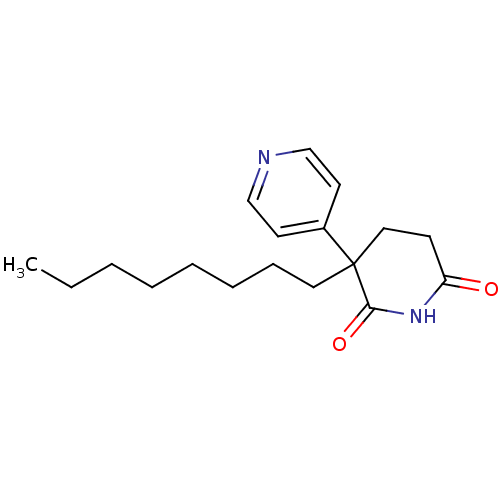
(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983
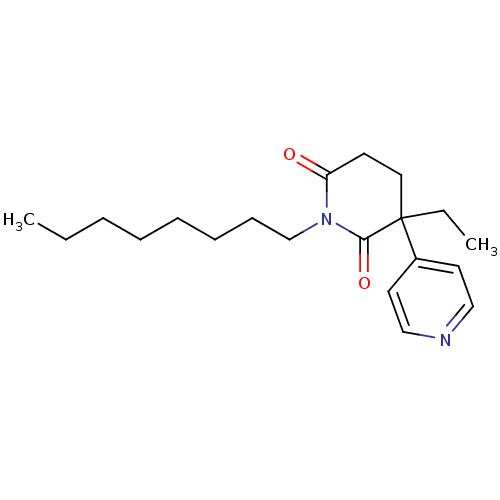
((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with testosterone |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50024544

(3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C18H26N2O2/c1-2-3-4-5-6-7-11-18(15-9-13-19-14-10-15)12-8-16(21)20-17(18)22/h9-10,13-14H,2-8,11-12H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015983

((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...)Show InChI InChI=1S/C20H30N2O2/c1-3-5-6-7-8-9-16-22-18(23)10-13-20(4-2,19(22)24)17-11-14-21-15-12-17/h11-12,14-15H,3-10,13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant (Ki) for Cytochrome P450 19A1 |
J Med Chem 28: 200-4 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1FK8 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8885
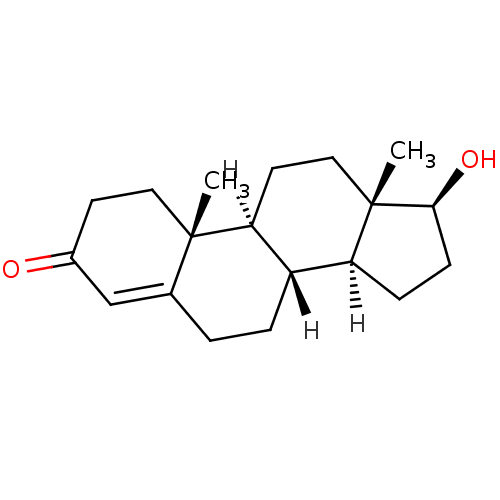
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase Cytochrome P450 19A1 |
J Med Chem 26: 50-4 (1983)
BindingDB Entry DOI: 10.7270/Q2V125CG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human placental cytochrome P450 19A1 |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant (Ki) for Cytochrome P450 19A1 |
J Med Chem 28: 200-4 (1985)
BindingDB Entry DOI: 10.7270/Q2WM1FK8 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50011760
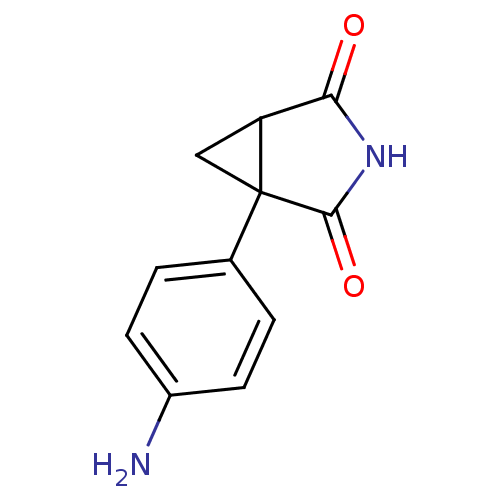
(1-(4-Amino-phenyl)-3-aza-bicyclo[3.1.0]hexane-2,4-...)Show InChI InChI=1S/C11H10N2O2/c12-7-3-1-6(2-4-7)11-5-8(11)9(14)13-10(11)15/h1-4,8H,5,12H2,(H,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 androstenedione |
J Med Chem 31: 971-6 (1988)
BindingDB Entry DOI: 10.7270/Q2154J7X |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with testosterone |
J Med Chem 31: 971-6 (1988)
BindingDB Entry DOI: 10.7270/Q2154J7X |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50228778

(4,4''-Dihydroxyoctafluoroazobenzene | CHEMBL79036)Show SMILES Oc1c(F)c(F)c(\N=N\c2c(F)c(F)c(O)c(F)c2F)c(F)c1F Show InChI InChI=1S/C12H2F8N2O2/c13-1-5(17)11(23)6(18)2(14)9(1)21-22-10-3(15)7(19)12(24)8(20)4(10)16/h23-24H/b22-21+ | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding constant of Testosterone-5 alpha-reductase activity at pH 7.4 |
J Med Chem 33: 2452-5 (1990)
BindingDB Entry DOI: 10.7270/Q2ZS2VGM |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50015844
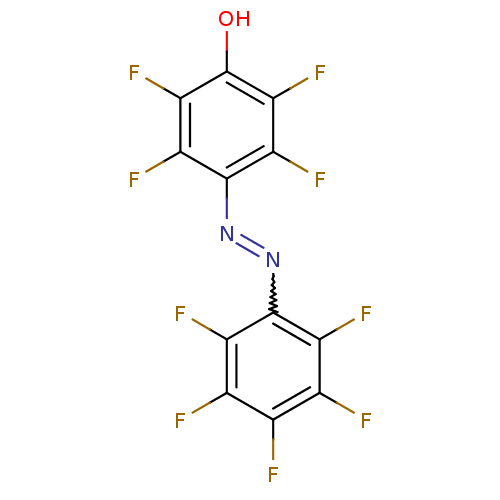
(2,3,5,6-Tetrafluoro-4-pentafluorophenylazo-phenol ...)Show SMILES Oc1c(F)c(F)c(N=Nc2c(F)c(F)c(F)c(F)c2F)c(F)c1F |w:8.8| Show InChI InChI=1S/C12HF9N2O/c13-1-2(14)4(16)10(5(17)3(1)15)22-23-11-6(18)8(20)12(24)9(21)7(11)19/h24H | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 17-alpha-hydroxylase/17,20 lyase from rat testes microsomal preparation |
J Med Chem 33: 2452-5 (1990)
BindingDB Entry DOI: 10.7270/Q2ZS2VGM |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50015985

(3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...)Show InChI InChI=1S/C12H14N2O2/c1-2-12(9-4-7-13-8-5-9)6-3-10(15)14-11(12)16/h4-5,7-8H,2-3,6H2,1H3,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytochrome P450 19A1 with androstenedione |
J Med Chem 30: 1550-4 (1987)
BindingDB Entry DOI: 10.7270/Q2GM869Q |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031677

((S)-13-Methyl-17-pyridin-3-yl-7,8,9,11,12,13,14,15...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1CC=C2c1cccnc1 |c:20| Show InChI InChI=1S/C23H25NO/c1-23-11-10-19-18-7-5-17(25)13-15(18)4-6-20(19)22(23)9-8-21(23)16-3-2-12-24-14-16/h2-3,5,7-8,12-14,19-20,22,25H,4,6,9-11H2,1H3/t19?,20?,22?,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052668
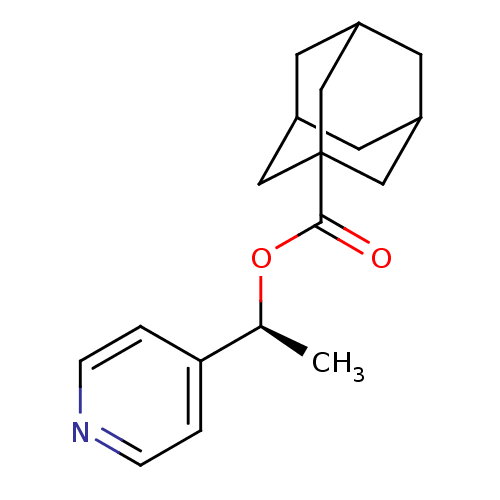
(Adamantane-1-carboxylic acid (S)-1-pyridin-4-yl-et...)Show SMILES C[C@H](OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:12:7:14:13.11.10,12:11:14:6.7.8,THB:10:11:6:14.9.8,10:9:6:13.11.12| Show InChI InChI=1S/C18H23NO2/c1-12(16-2-4-19-5-3-16)21-17(20)18-9-13-6-14(10-18)8-15(7-13)11-18/h2-5,12-15H,6-11H2,1H3/t12-,13?,14?,15?,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031666
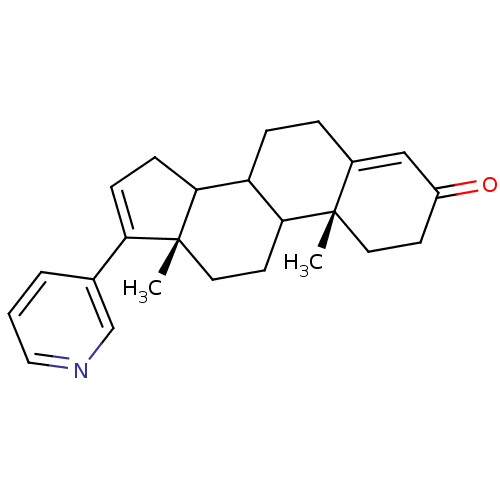
((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,6,7,8...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21,t:8| Show InChI InChI=1S/C24H29NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13-15,19,21-22H,5-6,8-12H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031669

((3R,5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-2,3...)Show SMILES C[C@]12CCC3C(CC[C@H]4C[C@H](O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H33NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17-19,21-22,26H,5-6,8-12,14H2,1-2H3/t17-,18+,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031677

((S)-13-Methyl-17-pyridin-3-yl-7,8,9,11,12,13,14,15...)Show SMILES C[C@]12CCC3C(CCc4cc(O)ccc34)C1CC=C2c1cccnc1 |c:20| Show InChI InChI=1S/C23H25NO/c1-23-11-10-19-18-7-5-17(25)13-15(18)4-6-20(19)22(23)9-8-21(23)16-3-2-12-24-14-16/h2-3,5,7-8,12-14,19-20,22,25H,4,6,9-11H2,1H3/t19?,20?,22?,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052669
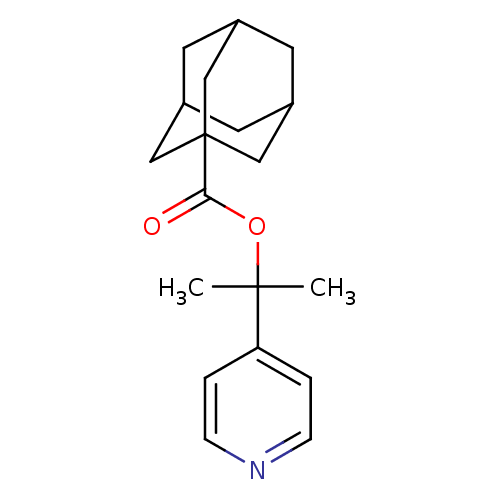
(Adamantane-1-carboxylic acid 1-methyl-1-pyridin-4-...)Show SMILES CC(C)(OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:9:10:14:7.8.13,13:8:15:14.12.11,13:12:15:7.8.9,THB:9:8:14:15.10.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-3-5-20-6-4-16)22-17(21)19-10-13-7-14(11-19)9-15(8-13)12-19/h3-6,13-15H,7-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of C17,20-lyase enzyme, cytochrome P450 17A1 in Human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031666

((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,6,7,8...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21,t:8| Show InChI InChI=1S/C24H29NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13-15,19,21-22H,5-6,8-12H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031665
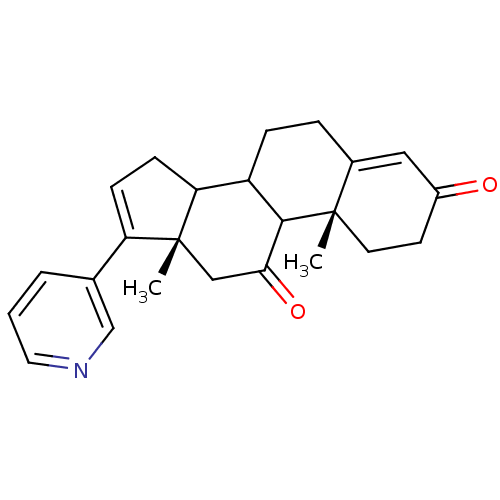
((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,6,7,8,9...)Show SMILES C[C@]12CC(=O)C3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:22,t:9| Show InChI InChI=1S/C24H27NO2/c1-23-10-9-17(26)12-16(23)5-6-18-20-8-7-19(15-4-3-11-25-14-15)24(20,2)13-21(27)22(18)23/h3-4,7,11-12,14,18,20,22H,5-6,8-10,13H2,1-2H3/t18?,20?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031666

((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,6,7,8...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21,t:8| Show InChI InChI=1S/C24H29NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13-15,19,21-22H,5-6,8-12H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone 17-alpha-hydroxylase. |
J Med Chem 41: 5375-81 (1999)
Article DOI: 10.1021/jm981017j
BindingDB Entry DOI: 10.7270/Q21C1XKW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031676
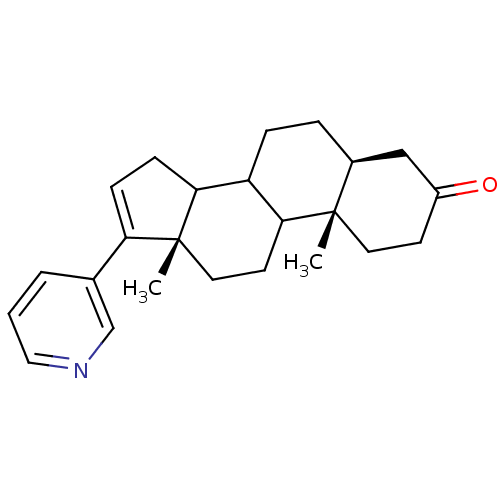
((5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,4,...)Show SMILES C[C@]12CCC3C(CC[C@H]4CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17,19,21-22H,5-6,8-12,14H2,1-2H3/t17-,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052668

(Adamantane-1-carboxylic acid (S)-1-pyridin-4-yl-et...)Show SMILES C[C@H](OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:12:7:14:13.11.10,12:11:14:6.7.8,THB:10:11:6:14.9.8,10:9:6:13.11.12| Show InChI InChI=1S/C18H23NO2/c1-12(16-2-4-19-5-3-16)21-17(20)18-9-13-6-14(10-18)8-15(7-13)11-18/h2-5,12-15H,6-11H2,1H3/t12-,13?,14?,15?,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone 17-alpha-hydroxylase. |
J Med Chem 41: 5375-81 (1999)
Article DOI: 10.1021/jm981017j
BindingDB Entry DOI: 10.7270/Q21C1XKW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM25458

((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...)Show SMILES [H][C@@]12CC=C(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |t:3,23| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-5,7,13,15,18-19,21-22,26H,6,8-12,14H2,1-2H3/t18-,19-,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031669

((3R,5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-2,3...)Show SMILES C[C@]12CCC3C(CC[C@H]4C[C@H](O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H33NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17-19,21-22,26H,5-6,8-12,14H2,1-2H3/t17-,18+,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031676

((5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,2,4,...)Show SMILES C[C@]12CCC3C(CC[C@H]4CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:21| Show InChI InChI=1S/C24H31NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,7,13,15,17,19,21-22H,5-6,8-12,14H2,1-2H3/t17-,19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029238
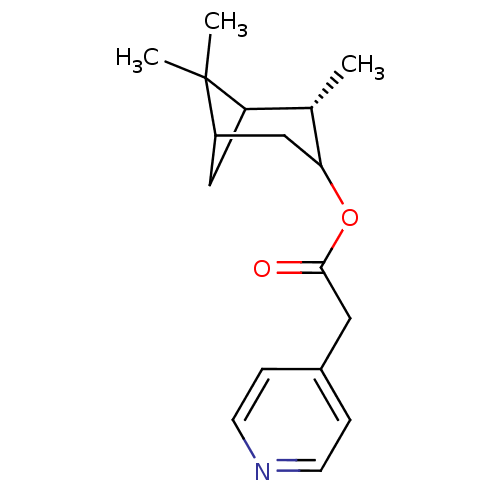
(CHEMBL132072 | Pyridin-4-yl-acetic acid (S)-2,6,6-...)Show SMILES C[C@H]1C2CC(CC1OC(=O)Cc1ccncc1)C2(C)C |TLB:7:6:17:3| Show InChI InChI=1S/C17H23NO2/c1-11-14-9-13(17(14,2)3)10-15(11)20-16(19)8-12-4-6-18-7-5-12/h4-7,11,13-15H,8-10H2,1-3H3/t11-,13?,14?,15?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50072799

((3S,5S,10S,13S)-10,13-Dimethyl-17-pyridin-3-yl-hex...)Show SMILES [H][C@@]12CCC(c3cccnc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@@H](O)CC[C@]12C Show InChI InChI=1S/C24H35NO/c1-23-11-9-18(26)14-17(23)5-6-19-21-8-7-20(16-4-3-13-25-15-16)24(21,2)12-10-22(19)23/h3-4,13,15,17-22,26H,5-12,14H2,1-2H3/t17-,18-,19-,20?,21-,22-,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone 17-alpha-hydroxylase. |
J Med Chem 41: 5375-81 (1999)
Article DOI: 10.1021/jm981017j
BindingDB Entry DOI: 10.7270/Q21C1XKW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031667

(3-((10R,13S)-10,13-Dimethyl-2,7,8,9,10,11,12,13,14...)Show SMILES C[C@]12CCC3C(CC=C4C=CCC[C@]34C)C1CC=C2c1cccnc1 |c:9,20,t:7| Show InChI InChI=1S/C24H29N/c1-23-13-4-3-7-18(23)8-9-19-21-11-10-20(17-6-5-15-25-16-17)24(21,2)14-12-22(19)23/h3,5-8,10,15-16,19,21-22H,4,9,11-14H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029237
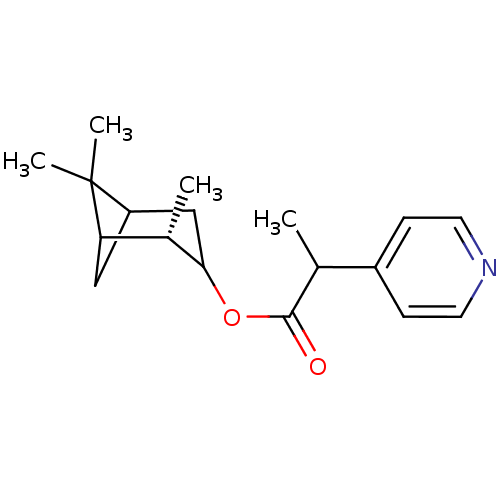
(2-Pyridin-4-yl-propionic acid (S)-2,6,6-trimethyl-...)Show SMILES CC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:4:5:12:8| Show InChI InChI=1S/C18H25NO2/c1-11(13-5-7-19-8-6-13)17(20)21-16-10-14-9-15(12(16)2)18(14,3)4/h5-8,11-12,14-16H,9-10H2,1-4H3/t11?,12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029237

(2-Pyridin-4-yl-propionic acid (S)-2,6,6-trimethyl-...)Show SMILES CC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:4:5:12:8| Show InChI InChI=1S/C18H25NO2/c1-11(13-5-7-19-8-6-13)17(20)21-16-10-14-9-15(12(16)2)18(14,3)4/h5-8,11-12,14-16H,9-10H2,1-4H3/t11?,12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50052669

(Adamantane-1-carboxylic acid 1-methyl-1-pyridin-4-...)Show SMILES CC(C)(OC(=O)C12CC3CC(CC(C3)C1)C2)c1ccncc1 |TLB:9:10:14:7.8.13,13:8:15:14.12.11,13:12:15:7.8.9,THB:9:8:14:15.10.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-3-5-20-6-4-16)22-17(21)19-10-13-7-14(11-19)9-15(8-13)12-19/h3-6,13-15H,7-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Campaign Centre for Cancer Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of 17-alpha-hydroxylase enzyme, cytochrome P450 17A1 of human testicular microsomes |
J Med Chem 39: 3319-23 (1996)
Article DOI: 10.1021/jm950749y
BindingDB Entry DOI: 10.7270/Q2KK99V3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029228
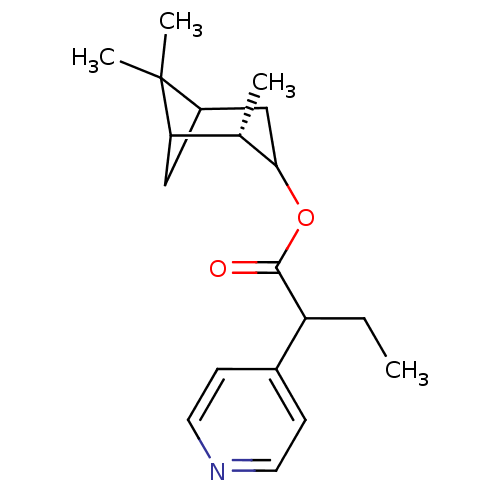
(2-Pyridin-4-yl-butyric acid (S)-2,6,6-trimethyl-bi...)Show SMILES CCC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:5:6:13:9| Show InChI InChI=1S/C19H27NO2/c1-5-15(13-6-8-20-9-7-13)18(21)22-17-11-14-10-16(12(17)2)19(14,3)4/h6-9,12,14-17H,5,10-11H2,1-4H3/t12-,14?,15?,16?,17?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029228

(2-Pyridin-4-yl-butyric acid (S)-2,6,6-trimethyl-bi...)Show SMILES CCC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1ccncc1 |TLB:5:6:13:9| Show InChI InChI=1S/C19H27NO2/c1-5-15(13-6-8-20-9-7-13)18(21)22-17-11-14-10-16(12(17)2)19(14,3)4/h6-9,12,14-17H,5,10-11H2,1-4H3/t12-,14?,15?,16?,17?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029240
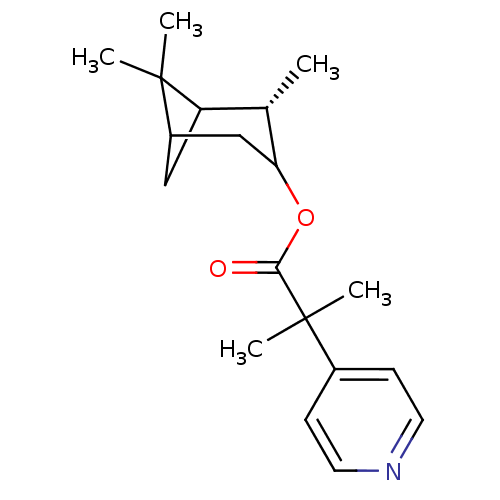
(2-Methyl-2-pyridin-4-yl-propionic acid (S)-2,6,6-t...)Show SMILES C[C@H]1C2CC(CC1OC(=O)C(C)(C)c1ccncc1)C2(C)C |TLB:7:6:19:3| Show InChI InChI=1S/C19H27NO2/c1-12-15-10-14(18(15,2)3)11-16(12)22-17(21)19(4,5)13-6-8-20-9-7-13/h6-9,12,14-16H,10-11H2,1-5H3/t12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031667

(3-((10R,13S)-10,13-Dimethyl-2,7,8,9,10,11,12,13,14...)Show SMILES C[C@]12CCC3C(CC=C4C=CCC[C@]34C)C1CC=C2c1cccnc1 |c:9,20,t:7| Show InChI InChI=1S/C24H29N/c1-23-13-4-3-7-18(23)8-9-19-21-11-10-20(17-6-5-15-25-16-17)24(21,2)14-12-22(19)23/h3,5-8,10,15-16,19,21-22H,4,9,11-14H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031665

((10R,13S)-10,13-Dimethyl-17-pyridin-3-yl-1,6,7,8,9...)Show SMILES C[C@]12CC(=O)C3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2c1cccnc1 |c:22,t:9| Show InChI InChI=1S/C24H27NO2/c1-23-10-9-17(26)12-16(23)5-6-18-20-8-7-19(15-4-3-11-25-14-15)24(20,2)13-21(27)22(18)23/h3-4,7,11-12,14,18,20,22H,5-6,8-10,13H2,1-2H3/t18?,20?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%. |
J Med Chem 38: 2463-71 (1995)
BindingDB Entry DOI: 10.7270/Q20K27KR |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029230
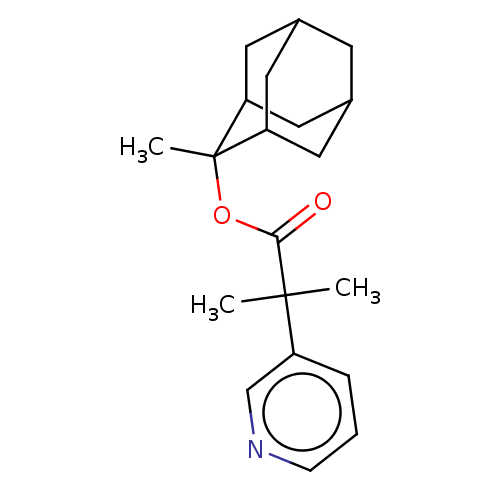
(2-Methyl-2-pyridin-3-yl-propionic acid 2-methyl-ad...)Show SMILES CC(C)(C(=O)OC1(C)C2CC3CC(C2)CC1C3)c1cccnc1 |TLB:7:6:10.9.16:12.13.14,5:6:13:10.9.11,16:15:13:10.9.11,THB:7:6:13:10.9.11,5:6:10.9.16:12.13.14,16:10:13:6.15.14,11:10:6:12.13.14,11:12:6:10.9.16,(4.95,-9.51,;4.66,-11.03,;4.37,-12.54,;6.18,-11.31,;6.68,-12.77,;7.18,-10.15,;8.69,-10.43,;8.91,-11.96,;10.07,-9.74,;10.07,-8.34,;10.91,-6.87,;12.28,-7.56,;12.28,-8.96,;11.44,-10.43,;10.91,-9.66,;9.53,-8.96,;9.53,-7.56,;3.15,-10.74,;2.64,-9.28,;1.13,-9,;.12,-10.16,;.63,-11.62,;2.14,-11.9,)| Show InChI InChI=1S/C20H27NO2/c1-19(2,15-5-4-6-21-12-15)18(22)23-20(3)16-8-13-7-14(10-16)11-17(20)9-13/h4-6,12-14,16-17H,7-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029229
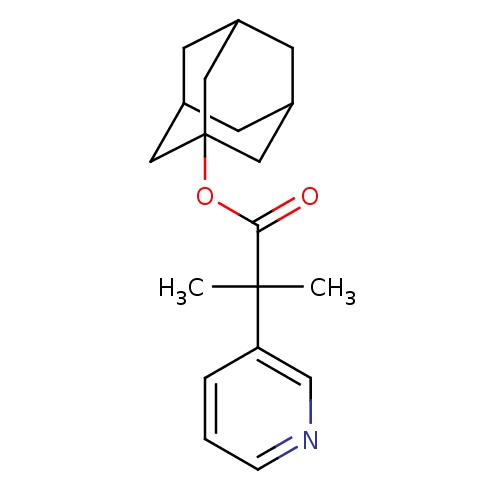
(2-Methyl-2-pyridin-3-yl-propionic acid adamantan-1...)Show SMILES CC(C)(C(=O)OC12CC3CC(CC(C3)C1)C2)c1cccnc1 |TLB:9:10:14:8.7.13,13:8:15:12.14.11,13:12:15:8.7.9,THB:9:8:14:10.15.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-4-3-5-20-12-16)17(21)22-19-9-13-6-14(10-19)8-15(7-13)11-19/h3-5,12-15H,6-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029229

(2-Methyl-2-pyridin-3-yl-propionic acid adamantan-1...)Show SMILES CC(C)(C(=O)OC12CC3CC(CC(C3)C1)C2)c1cccnc1 |TLB:9:10:14:8.7.13,13:8:15:12.14.11,13:12:15:8.7.9,THB:9:8:14:10.15.11| Show InChI InChI=1S/C19H25NO2/c1-18(2,16-4-3-5-20-12-16)17(21)22-19-9-13-6-14(10-19)8-15(7-13)11-19/h3-5,12-15H,6-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50031667

(3-((10R,13S)-10,13-Dimethyl-2,7,8,9,10,11,12,13,14...)Show SMILES C[C@]12CCC3C(CC=C4C=CCC[C@]34C)C1CC=C2c1cccnc1 |c:9,20,t:7| Show InChI InChI=1S/C24H29N/c1-23-13-4-3-7-18(23)8-9-19-21-11-10-20(17-6-5-15-25-16-17)24(21,2)14-12-22(19)23/h3,5-8,10,15-16,19,21-22H,4,9,11-14H2,1-2H3/t19?,21?,22?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone 17-alpha-hydroxylase. |
J Med Chem 41: 5375-81 (1999)
Article DOI: 10.1021/jm981017j
BindingDB Entry DOI: 10.7270/Q21C1XKW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50029241
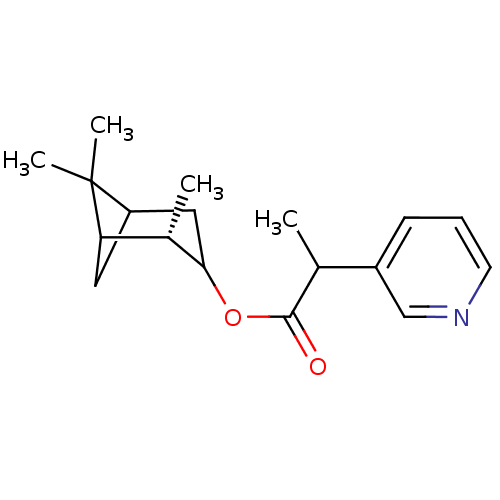
(2-Pyridin-3-yl-propionic acid (S)-2,6,6-trimethyl-...)Show SMILES CC(C(=O)OC1CC2CC([C@@H]1C)C2(C)C)c1cccnc1 |TLB:4:5:12:8| Show InChI InChI=1S/C18H25NO2/c1-11(13-6-5-7-19-10-13)17(20)21-16-9-14-8-15(12(16)2)18(14,3)4/h5-7,10-12,14-16H,8-9H2,1-4H3/t11?,12-,14?,15?,16?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CRC Laboratory
Curated by ChEMBL
| Assay Description
Tested for inhibition of human testicular C17,20-Lyase. |
J Med Chem 38: 4191-7 (1995)
BindingDB Entry DOI: 10.7270/Q2NK3D2C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data