| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5'-AMP-activated protein kinase catalytic subunit alpha-1 |
|---|
| Ligand | BDBM25118 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_586314 (CHEMBL1060175) |
|---|
| Kd | 520±n/a nM |
|---|
| Citation |  Karaman, MW; Herrgard, S; Treiber, DK; Gallant, P; Atteridge, CE; Campbell, BT; Chan, KW; Ciceri, P; Davis, MI; Edeen, PT; Faraoni, R; Floyd, M; Hunt, JP; Lockhart, DJ; Milanov, ZV; Morrison, MJ; Pallares, G; Patel, HK; Pritchard, S; Wodicka, LM; Zarrinkar, PP A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol26:127-32 (2008) [PubMed] Article Karaman, MW; Herrgard, S; Treiber, DK; Gallant, P; Atteridge, CE; Campbell, BT; Chan, KW; Ciceri, P; Davis, MI; Edeen, PT; Faraoni, R; Floyd, M; Hunt, JP; Lockhart, DJ; Milanov, ZV; Morrison, MJ; Pallares, G; Patel, HK; Pritchard, S; Wodicka, LM; Zarrinkar, PP A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol26:127-32 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5'-AMP-activated protein kinase catalytic subunit alpha-1 |
|---|
| Name: | 5'-AMP-activated protein kinase catalytic subunit alpha-1 |
|---|
| Synonyms: | AAPK1_HUMAN | ACACA kinase | AMP-activated protein kinase alpha-1/beta-2/gamma-2 | AMP-activated protein kinase, alpha-1 subunit | AMPK subunit alpha-1 | AMPK-alpha1 | AMPK1 | Hydroxymethylglutaryl-CoA reductase kinase | PRKAA1 | Tau-protein kinase PRKAA1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 64023.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q13131 |
|---|
| Residue: | 559 |
|---|
| Sequence: | MRRLSSWRKMATAEKQKHDGRVKIGHYILGDTLGVGTFGKVKVGKHELTGHKVAVKILNR
QKIRSLDVVGKIRREIQNLKLFRHPHIIKLYQVISTPSDIFMVMEYVSGGELFDYICKNG
RLDEKESRRLFQQILSGVDYCHRHMVVHRDLKPENVLLDAHMNAKIADFGLSNMMSDGEF
LRTSCGSPNYAAPEVISGRLYAGPEVDIWSSGVILYALLCGTLPFDDDHVPTLFKKICDG
IFYTPQYLNPSVISLLKHMLQVDPMKRATIKDIREHEWFKQDLPKYLFPEDPSYSSTMID
DEALKEVCEKFECSEEEVLSCLYNRNHQDPLAVAYHLIIDNRRIMNEAKDFYLATSPPDS
FLDDHHLTRPHPERVPFLVAETPRARHTLDELNPQKSKHQGVRKAKWHLGIRSQSRPNDI
MAEVCRAIKQLDYEWKVVNPYYLRVRRKNPVTSTYSKMSLQLYQVDSRTYLLDFRSIDDE
ITEAKSGTATPQRSGSVSNYRSCQRSDSDAEAQGKSSEVSLTSSVTSLDSSPVDLTPRPG
SHTIEFFEMCANLIKILAQ
|
|
|
|---|
| BDBM25118 |
|---|
| n/a |
|---|
| Name | BDBM25118 |
|---|
| Synonyms: | (3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,3-dihydrobenzimidazol-2-ylidene]carbostyril | 4-amino-5-fluoro-3-[6-(4-methylpiperazin-1-yl)-1H-1,3-benzodiazol-2-yl]-1,2-dihydroquinolin-2-one | CHEMBL522892 | CHIR-258 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H21FN6O |
|---|
| Mol. Mass. | 392.4294 |
|---|
| SMILES | CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(F)cccc2[nH]c1=O |
|---|
| Structure | 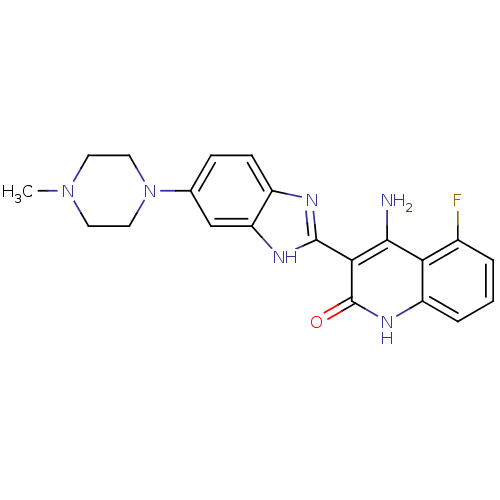
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Karaman, MW; Herrgard, S; Treiber, DK; Gallant, P; Atteridge, CE; Campbell, BT; Chan, KW; Ciceri, P; Davis, MI; Edeen, PT; Faraoni, R; Floyd, M; Hunt, JP; Lockhart, DJ; Milanov, ZV; Morrison, MJ; Pallares, G; Patel, HK; Pritchard, S; Wodicka, LM; Zarrinkar, PP A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol26:127-32 (2008) [PubMed] Article
Karaman, MW; Herrgard, S; Treiber, DK; Gallant, P; Atteridge, CE; Campbell, BT; Chan, KW; Ciceri, P; Davis, MI; Edeen, PT; Faraoni, R; Floyd, M; Hunt, JP; Lockhart, DJ; Milanov, ZV; Morrison, MJ; Pallares, G; Patel, HK; Pritchard, S; Wodicka, LM; Zarrinkar, PP A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol26:127-32 (2008) [PubMed] Article