

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM12255 (AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50394785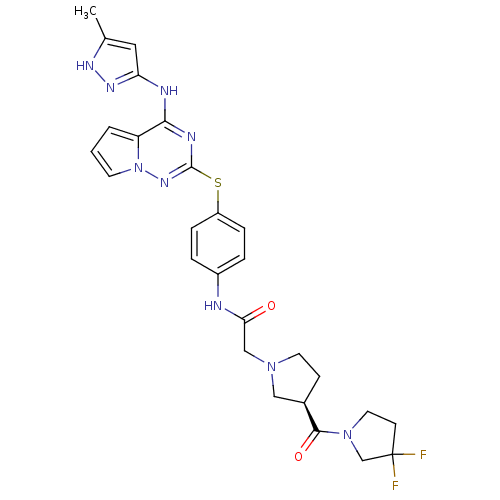 (CHEMBL2163404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM25118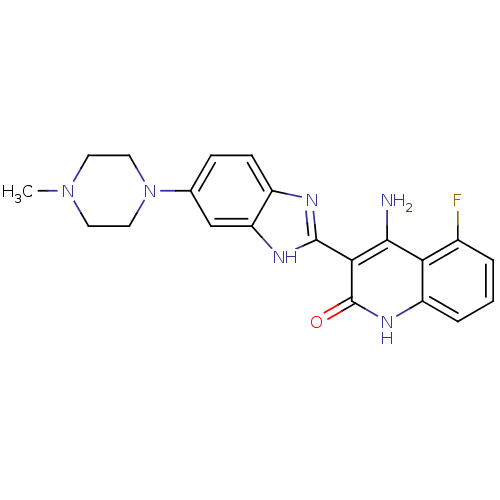 ((3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM185674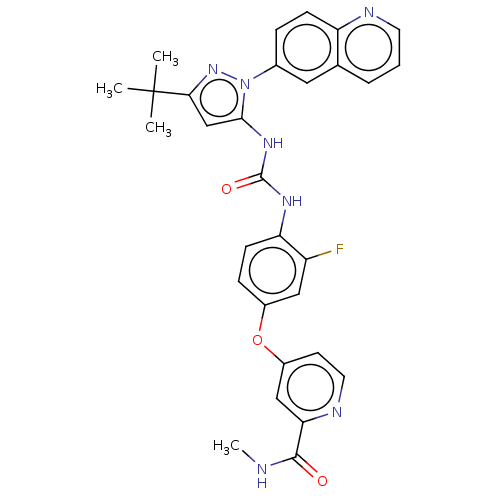 (4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50394779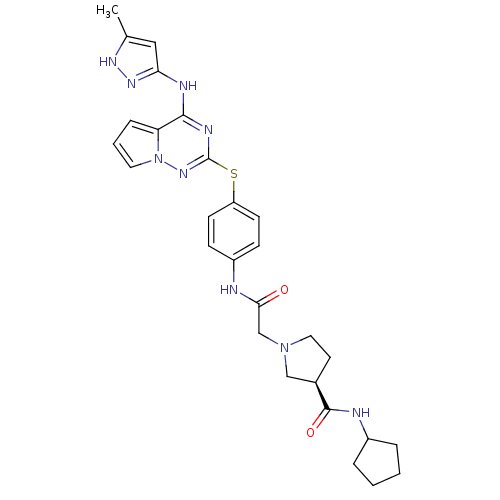 (CHEMBL2163394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase A autophosphorylation in human HEK293 cells after 2 hrs by phosphor antibody readout assay | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM185674 (4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394779 (CHEMBL2163394) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394785 (CHEMBL2163404) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM12255 (AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394798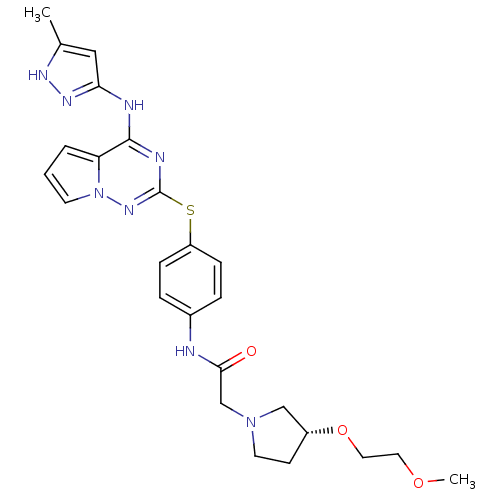 (CHEMBL2163408) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394777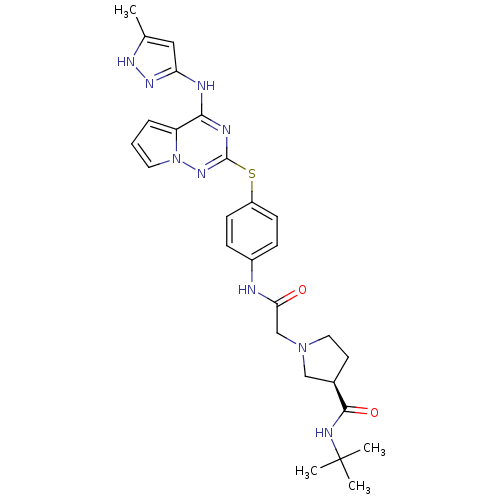 (CHEMBL2163387) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394786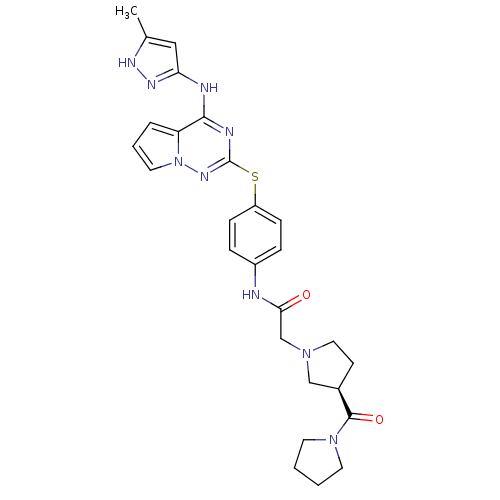 (CHEMBL2163403) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394776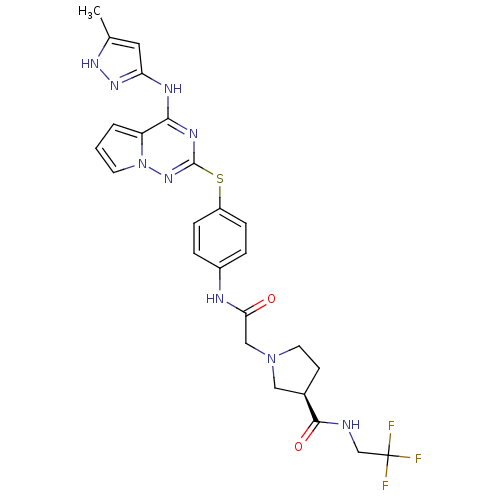 (CHEMBL2163388) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM14948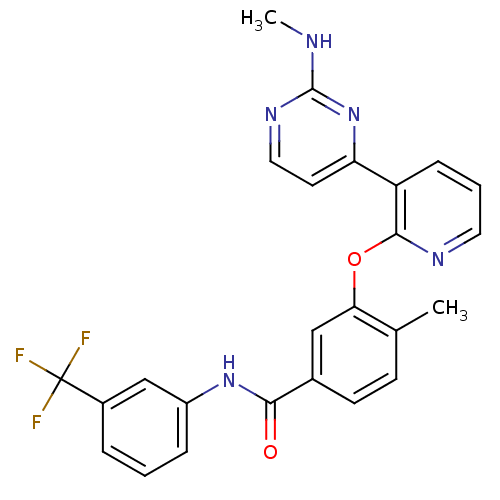 (4-Methyl-3-(3-(2-(methylamino)pyrimidin-4-yl)pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394794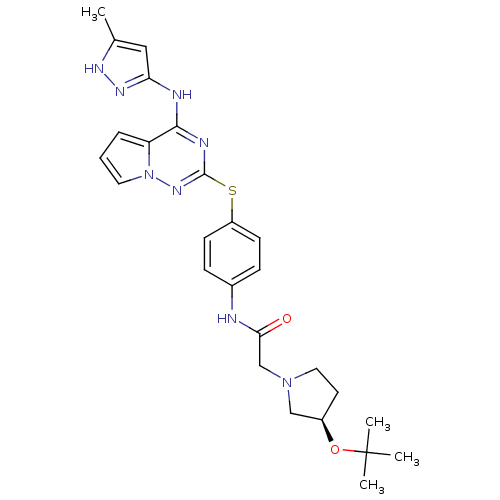 (CHEMBL2163412) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394786 (CHEMBL2163403) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394778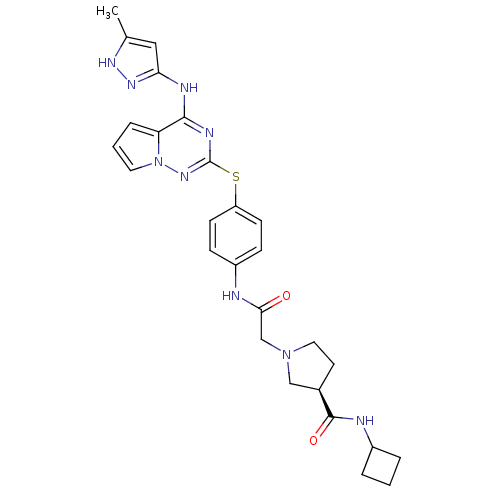 (CHEMBL2163395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394784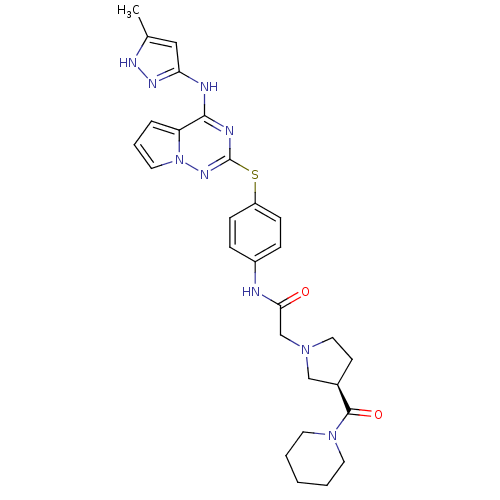 (CHEMBL2163389) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394789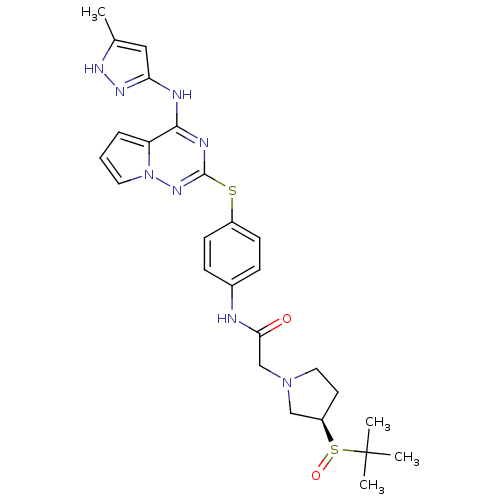 (CHEMBL2163400) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM26105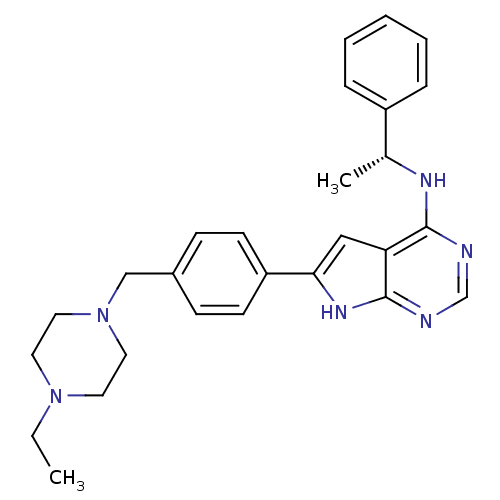 (6-{4-[(4-ethylpiperazin-1-yl)methyl]phenyl}-N-[(1R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394799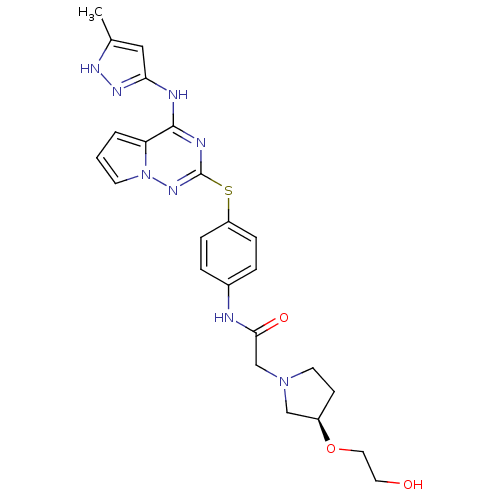 (CHEMBL2163407) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394783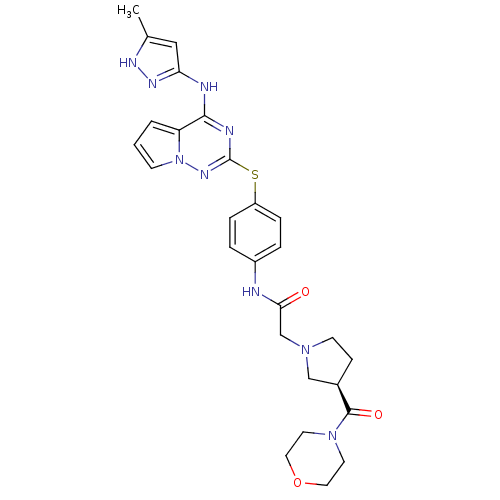 (CHEMBL2163390) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM26105 (6-{4-[(4-ethylpiperazin-1-yl)methyl]phenyl}-N-[(1R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM14948 (4-Methyl-3-(3-(2-(methylamino)pyrimidin-4-yl)pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394795 (CHEMBL2163411) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394788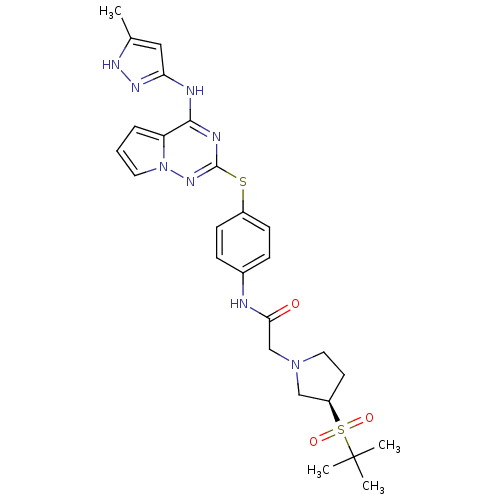 (CHEMBL2163401) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM228659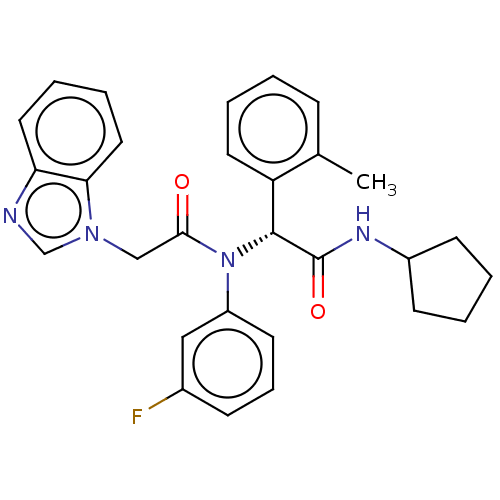 ((+)-2-(2-(1H-Benzo[d]imidazol-1-yl)-N-(3-fluorophe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The activity of IDH1 R132H and IDH1 R132C was measured in 384-well plates by coupling NADPH consumption to a diaphorase/resazurin-based detection sys... | J Biol Chem 289: 13717-25 (2014) Article DOI: 10.1074/jbc.M113.511030 BindingDB Entry DOI: 10.7270/Q2513X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50262685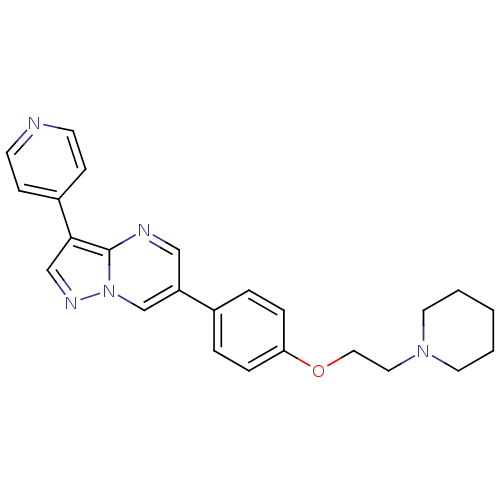 (6-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM25118 ((3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567821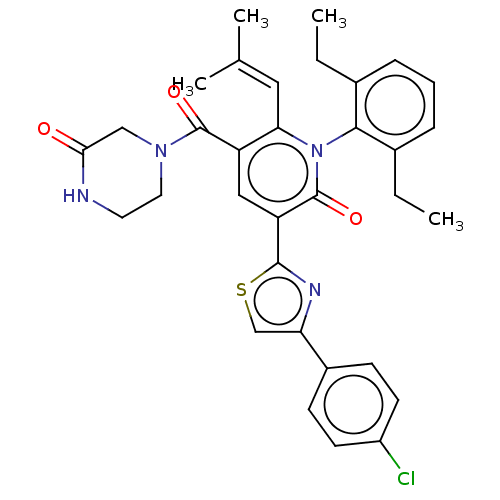 (CHEMBL4875277) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132H mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394787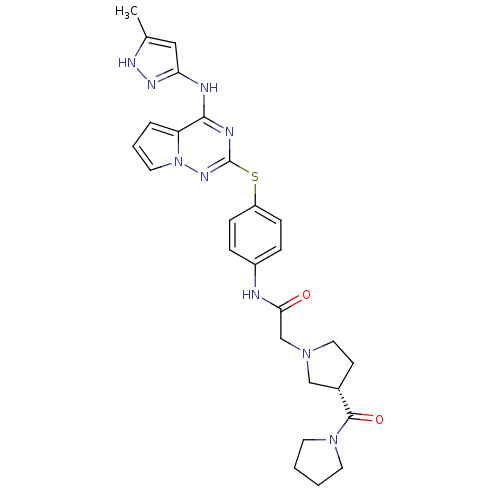 (CHEMBL2163402) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567816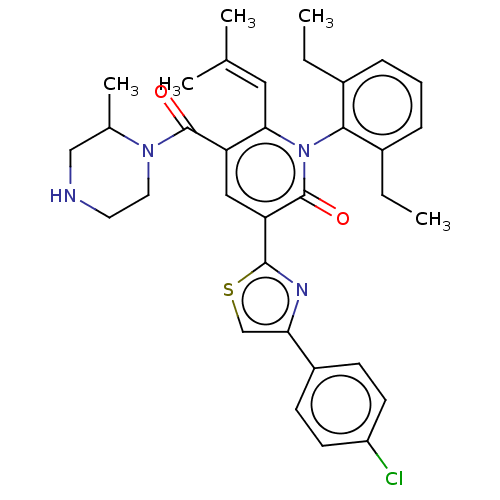 (CHEMBL4851708) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132H mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567821 (CHEMBL4875277) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132C mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM50392788 (CHEMBL457614) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-phosphatidylinositol 3-phosphate 5-kinase (Homo sapiens) | BDBM50511422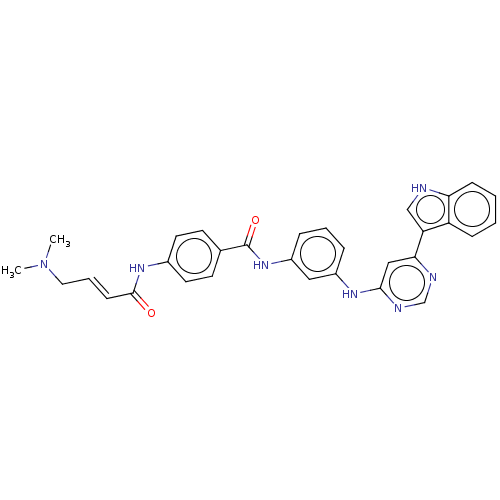 (CHEMBL4446338) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged full length human PIKFYVE (1 to 2098 residues) using PI(3)P and Phosphatidylserine as substrate by ADP-Glo assay | ACS Med Chem Lett 11: 346-352 (2020) Article DOI: 10.1021/acsmedchemlett.9b00402 BindingDB Entry DOI: 10.7270/Q2TM7FDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567825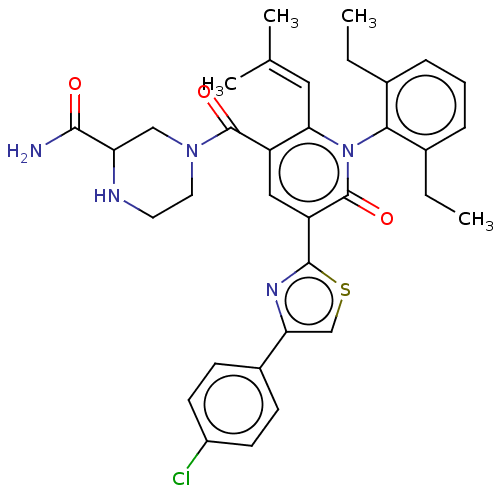 (CHEMBL4861426) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132C mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394780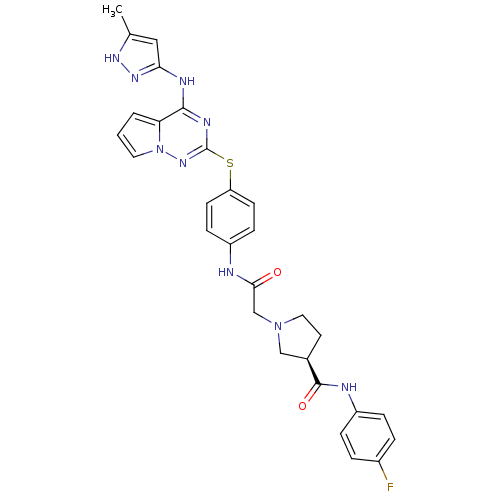 (CHEMBL2163393) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394797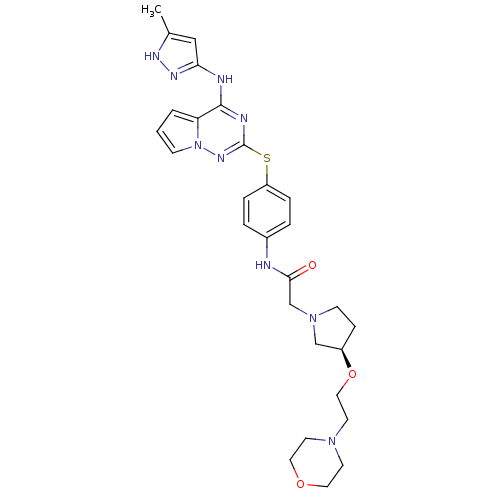 (CHEMBL2163409) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567808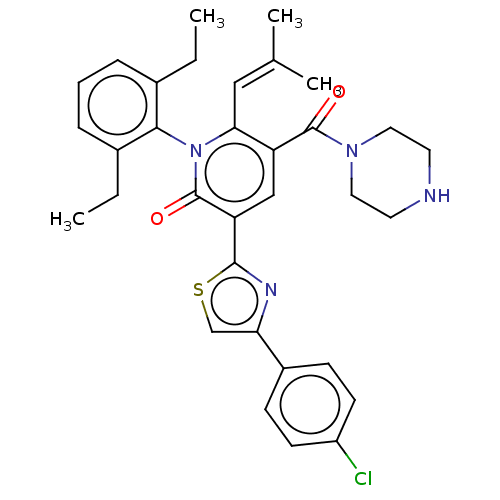 (CHEMBL4860083) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132H mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567815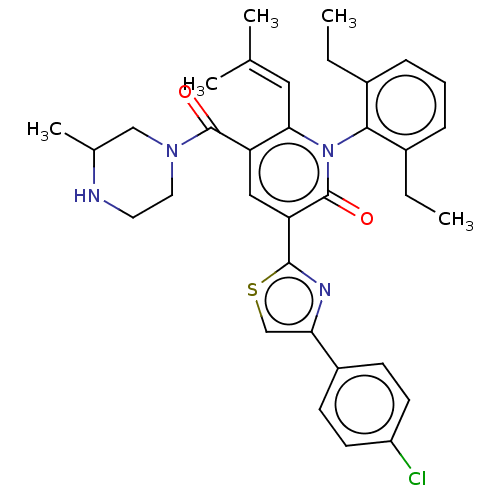 (CHEMBL4864243) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132H mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567832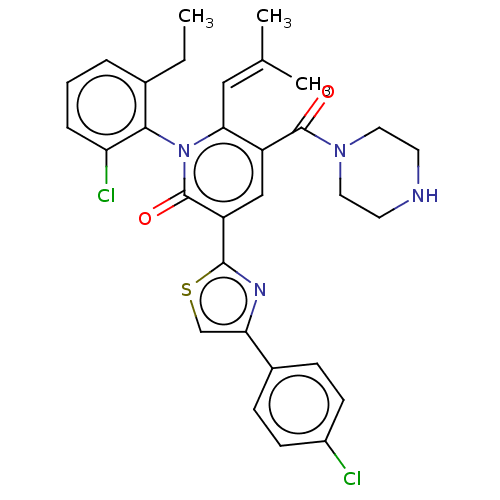 (CHEMBL4878489) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132H mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50352318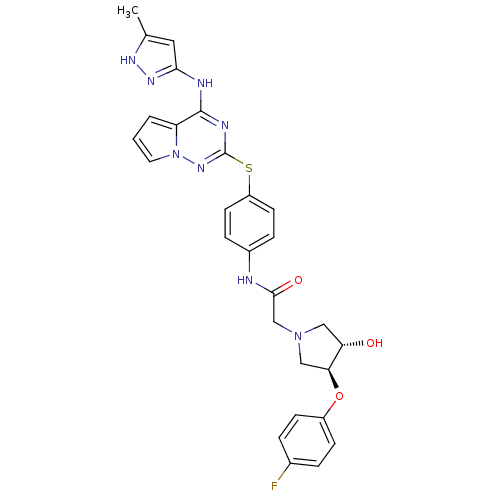 (CHEMBL1822658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567833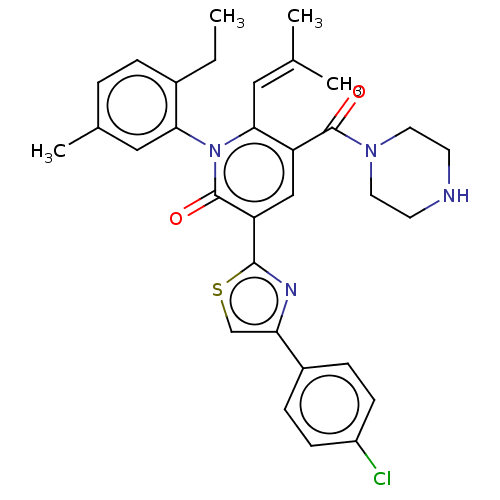 (CHEMBL4868649) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132H mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50394791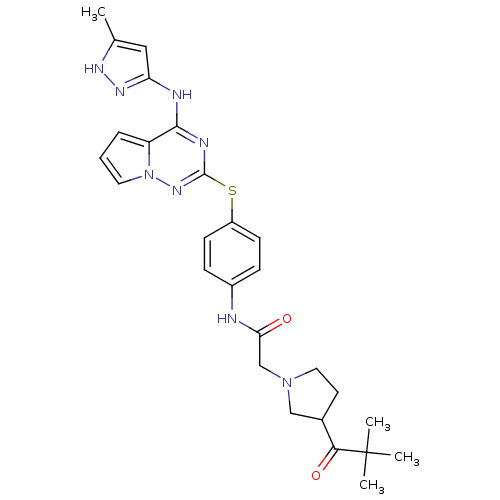 (CHEMBL2163397) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Corporation Curated by ChEMBL | Assay Description Inhibition of Aurora kinase B-mediated histone H3 Ser10 phosphorylation in human HCT116 cells after 2 hrs by sandwich ELISA | J Med Chem 55: 3250-60 (2012) Article DOI: 10.1021/jm201702g BindingDB Entry DOI: 10.7270/Q2W0971F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567815 (CHEMBL4864243) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132C mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567828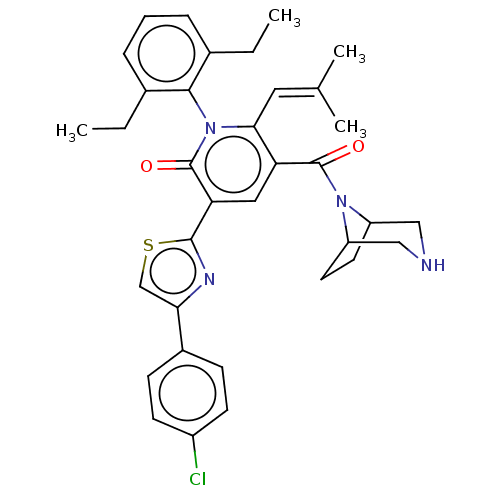 (CHEMBL4854924) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132C mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50567819 (CHEMBL4845768) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDH1 R132C mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate incubated for 5 mins by diaph... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00019 BindingDB Entry DOI: 10.7270/Q2FF3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 44219 total ) | Next | Last >> |