| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 3A |
|---|
| Ligand | BDBM50060685 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_592422 (CHEMBL1037808) |
|---|
| Kd | 64.57±n/a nM |
|---|
| Citation |  Butini, S; Budriesi, R; Hamon, M; Morelli, E; Gemma, S; Brindisi, M; Borrelli, G; Novellino, E; Fiorini, I; Ioan, P; Chiarini, A; Cagnotto, A; Mennini, T; Fracasso, C; Caccia, S; Campiani, G Novel, potent, and selective quinoxaline-based 5-HT(3) receptor ligands. 1. Further structure-activity relationships and pharmacological characterization. J Med Chem52:6946-50 (2009) [PubMed] Article Butini, S; Budriesi, R; Hamon, M; Morelli, E; Gemma, S; Brindisi, M; Borrelli, G; Novellino, E; Fiorini, I; Ioan, P; Chiarini, A; Cagnotto, A; Mennini, T; Fracasso, C; Caccia, S; Campiani, G Novel, potent, and selective quinoxaline-based 5-HT(3) receptor ligands. 1. Further structure-activity relationships and pharmacological characterization. J Med Chem52:6946-50 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 3A |
|---|
| Name: | 5-hydroxytryptamine receptor 3A |
|---|
| Synonyms: | 5-HT3 | 5-hydroxytryptamine receptor 3A | 5HT3A_CAVPO | 5HT3R | HTR3 | HTR3A | Serotonin 3a (5-HT3a) receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 55657.07 |
|---|
| Organism: | GUINEA PIG |
|---|
| Description: | 5-HT3 HTR3A GUINEA PIG::O70212 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MVLWLQLALLALLLPTSLAQGEVRGKGTAQAHNSTRPALQRLSDHLLADYRKSVRPVRDW
RKPTTVSIDAIVYAILSVDEKNQVLTTYIWYRQFWTDEFLQWNPEDFDNITKLSIPTDSI
WVPDILINEFVDVGKSPNIPYVYVRHQGEVQNYKPLQVVTACSLDIYNFPFDVQNCSLTF
TSWLHTIQDINISLWRLPEKVKSDKSVFMNQGEWELLGVLTEFLEFSDRESRGSFAEMKF
YVVIRRRPLFYAVTLLLPSIFLMIVDIVGFYLPPDSGERVSFKITLLLGYSVFLIIVSDT
LPATAIGTPLISVYFVVCMALLVISLAETILIVRLVHKQDLQQPVPLWLRHLVLERIAGL
LCLGEQLTSHRGPATLQATKTDDFSGSTLLPAMGNHCGPLGGPQDLEKTSRGRGSPPPPP
REASLAMCGLLQELASIRHFLEKREETREVARDWLRVGSVLDKLLFRVYLLAVLAYSITL
VTLWSVWHYA
|
|
|
|---|
| BDBM50060685 |
|---|
| n/a |
|---|
| Name | BDBM50060685 |
|---|
| Synonyms: | 9-Fluoro-4-(4-methyl-piperazin-1-yl)-pyrrolo[1,2-a]quinoxaline | 9-fluoro-4-(4-methylpiperazin-1-yl)pyrrolo[1,2-a]quinoxaline | CHEMBL124886 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H17FN4 |
|---|
| Mol. Mass. | 284.3314 |
|---|
| SMILES | CN1CCN(CC1)c1nc2cccc(F)c2n2cccc12 |
|---|
| Structure | 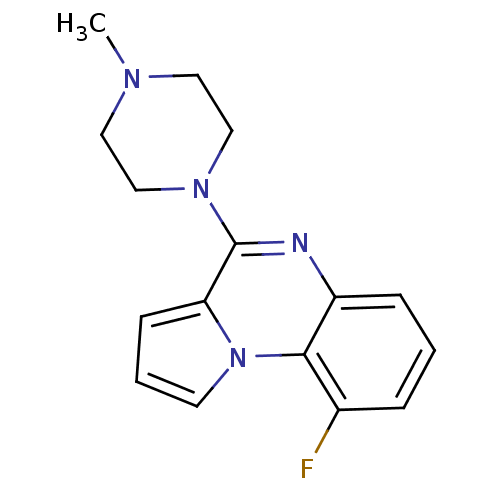
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Butini, S; Budriesi, R; Hamon, M; Morelli, E; Gemma, S; Brindisi, M; Borrelli, G; Novellino, E; Fiorini, I; Ioan, P; Chiarini, A; Cagnotto, A; Mennini, T; Fracasso, C; Caccia, S; Campiani, G Novel, potent, and selective quinoxaline-based 5-HT(3) receptor ligands. 1. Further structure-activity relationships and pharmacological characterization. J Med Chem52:6946-50 (2009) [PubMed] Article
Butini, S; Budriesi, R; Hamon, M; Morelli, E; Gemma, S; Brindisi, M; Borrelli, G; Novellino, E; Fiorini, I; Ioan, P; Chiarini, A; Cagnotto, A; Mennini, T; Fracasso, C; Caccia, S; Campiani, G Novel, potent, and selective quinoxaline-based 5-HT(3) receptor ligands. 1. Further structure-activity relationships and pharmacological characterization. J Med Chem52:6946-50 (2009) [PubMed] Article