

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271367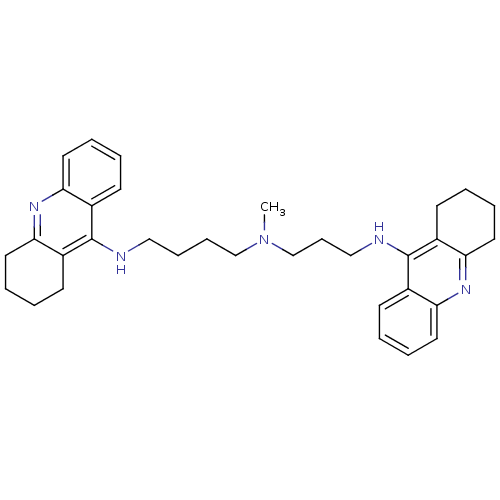 (CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271367 (CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50271556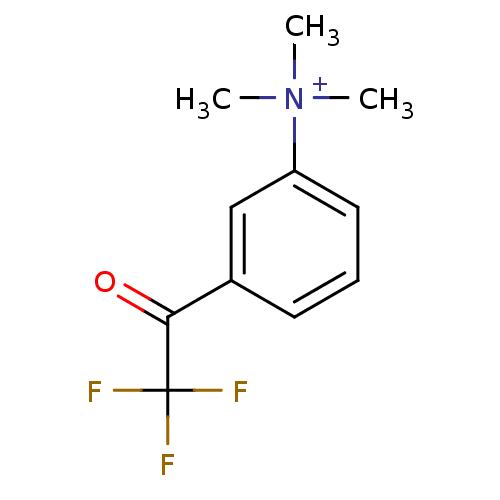 (CHEMBL525622 | N,N,N-trimethyl-3-(2,2,2-trifluoroa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369748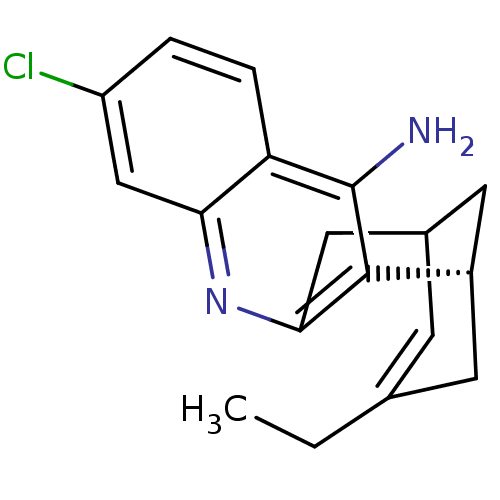 (CHEMBL208599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibitory activity against human AChE | J Med Chem 46: 1-4 (2002) Article DOI: 10.1021/jm0255668 BindingDB Entry DOI: 10.7270/Q2GF0V7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10597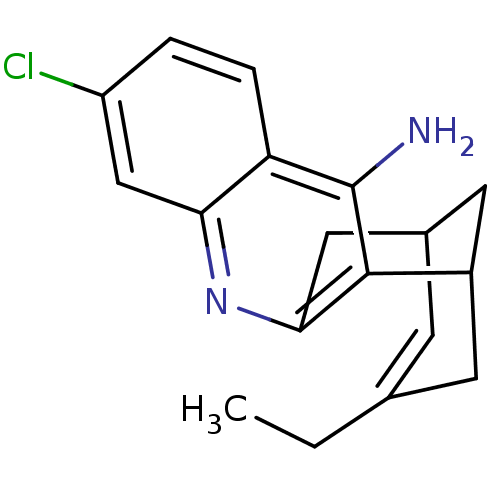 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... | J Med Chem 49: 3421-5 (2006) Article DOI: 10.1021/jm060257t BindingDB Entry DOI: 10.7270/Q2WW7FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50370598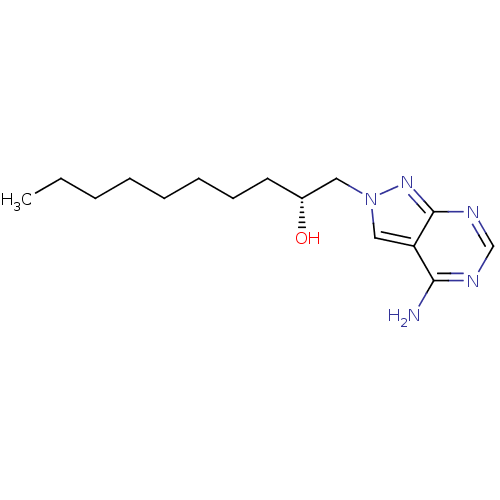 (CHEMBL1651379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibitory constant against bovine spleen Adenosine deaminase | J Med Chem 48: 5162-74 (2005) Article DOI: 10.1021/jm050136d BindingDB Entry DOI: 10.7270/Q2542PCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50005193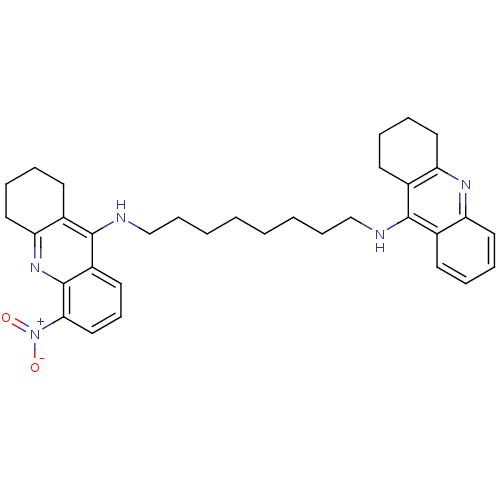 (CHEMBL3099496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human butyrylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8965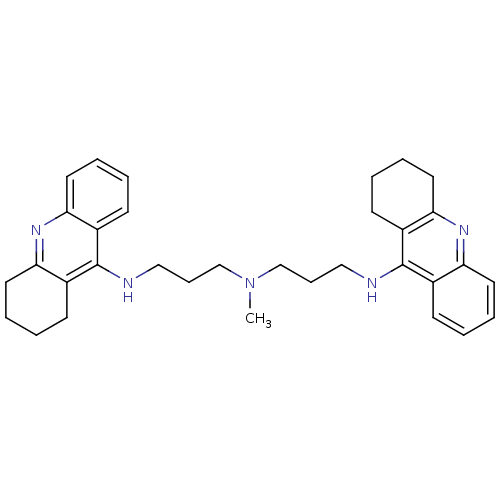 (CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of fetal Bovine serum AChE | J Med Chem 46: 1-4 (2002) Article DOI: 10.1021/jm0255668 BindingDB Entry DOI: 10.7270/Q2GF0V7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8965 (CHEMBL338755 | Tacrine Dimer 4a | methylbis[3-(1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -58.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50149201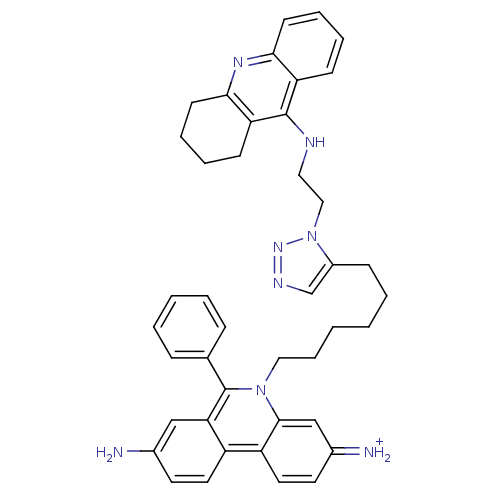 (3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271469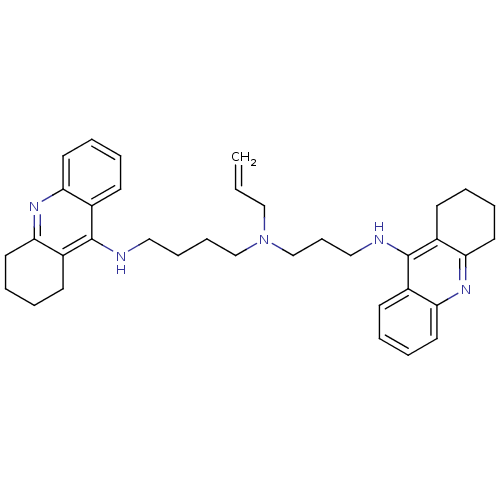 (CHEMBL507174 | N-Allyl-N-(1,2,3,4-tetrahydroacridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001107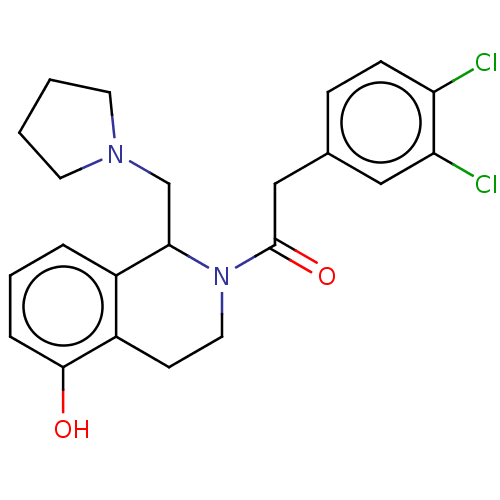 (2-(3,4-Dichloro-phenyl)-1-(5-hydroxy-1-pyrrolidin-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 in guinea pig brain homogenates | J Med Chem 43: 2124-34 (2000) BindingDB Entry DOI: 10.7270/Q23N242D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50265775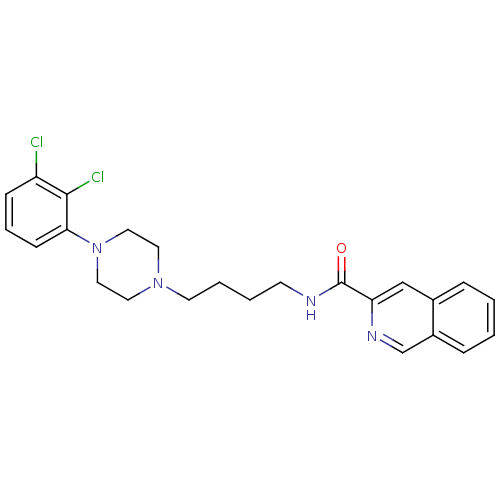 (CHEMBL462508 | N-[4-[4-(2,3-Dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry | J Med Chem 52: 151-69 (2009) Article DOI: 10.1021/jm800689g BindingDB Entry DOI: 10.7270/Q2J67GSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118528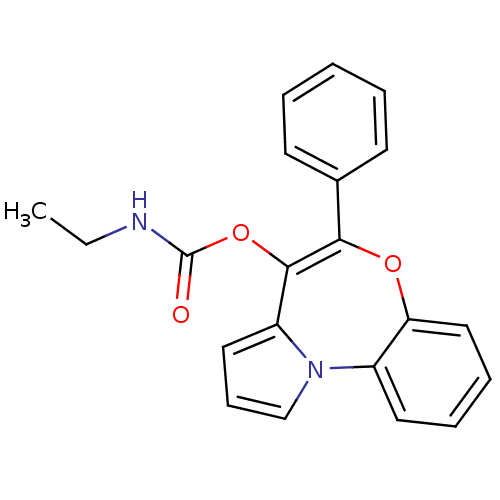 (CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-5-4864 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50265375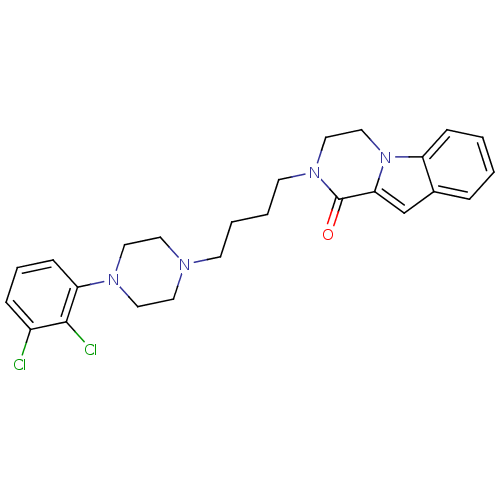 (CHEMBL496531 | N-[4-[4-(2,3-Dichlorophenyl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry | J Med Chem 52: 151-69 (2009) Article DOI: 10.1021/jm800689g BindingDB Entry DOI: 10.7270/Q2J67GSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8977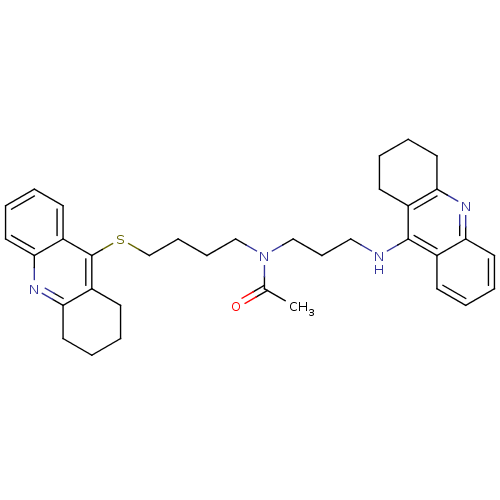 (CHEMBL175949 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118528 (CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271470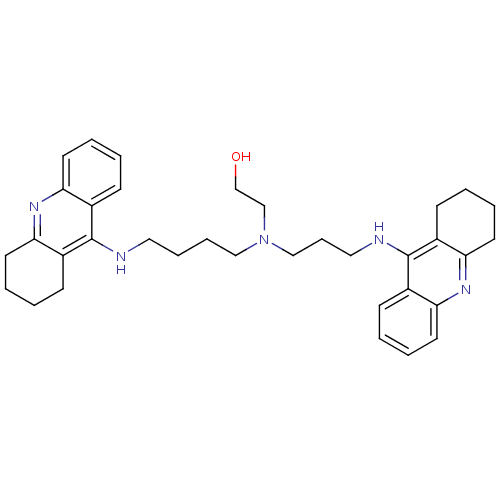 (CHEMBL499224 | N-(2-Hydroxyethyl)-N-(1,2,3,4-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177895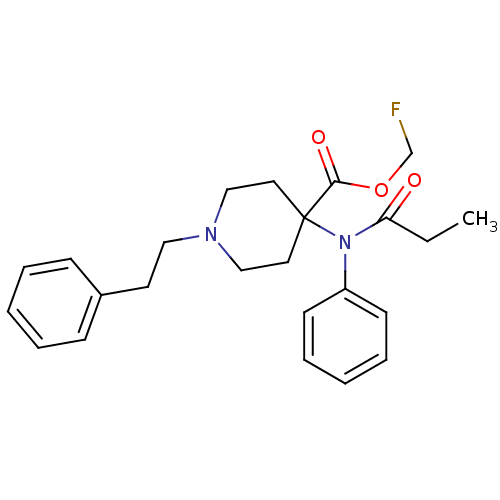 (CHEMBL200456 | fluoromethyl 1-(2-phenylethyl)-4-(N...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50177898 (2-Fluoro-N-[4-methoxymethyl-1-(2-thiophen-2-yl-eth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität München Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor | J Med Chem 48: 7720-32 (2005) Article DOI: 10.1021/jm0507274 BindingDB Entry DOI: 10.7270/Q2G44PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50171394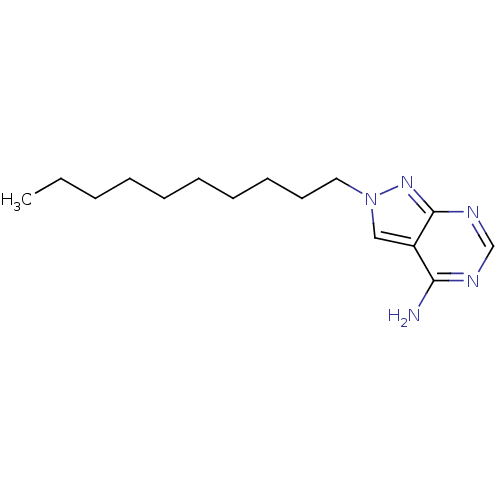 (2-Decyl-2H-pyrazolo[3,4-d]pyrimidin-4-ylamine | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibitory constant against bovine spleen Adenosine deaminase | J Med Chem 48: 5162-74 (2005) Article DOI: 10.1021/jm050136d BindingDB Entry DOI: 10.7270/Q2542PCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50369748 (CHEMBL208599) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271471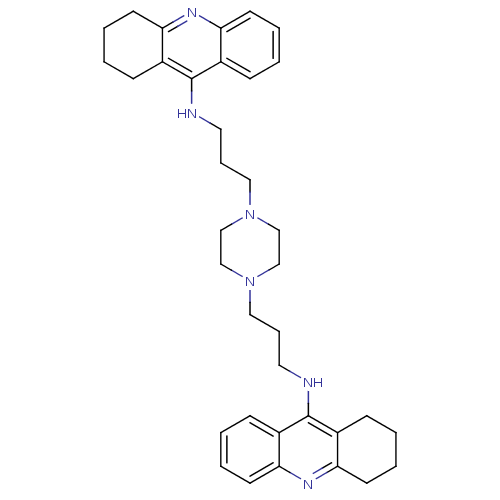 (1,4-bis[3-(1,2,3,4-Tetrahydroacridin-9-yl)aminopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50265445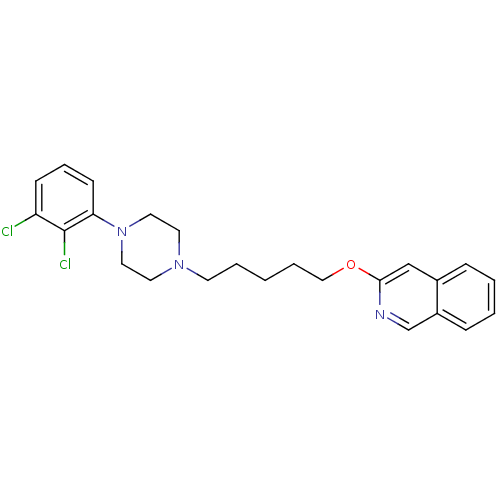 (3-[5-[4-(2,3-Dichlorophenyl)piperazin-1-yl]pentylo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry | J Med Chem 52: 151-69 (2009) Article DOI: 10.1021/jm800689g BindingDB Entry DOI: 10.7270/Q2J67GSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to 5HT2A receptor (unknown origin) | J Med Chem 57: 9578-97 (2014) Article DOI: 10.1021/jm501119j BindingDB Entry DOI: 10.7270/Q2571DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry | J Med Chem 52: 151-69 (2009) Article DOI: 10.1021/jm800689g BindingDB Entry DOI: 10.7270/Q2J67GSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50253857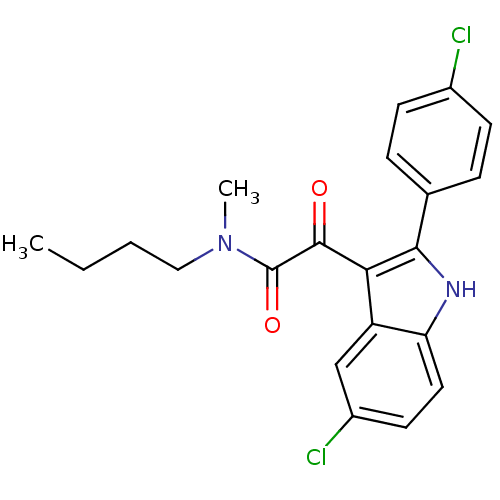 (CHEMBL460998 | N-butyl-2-(5-chloro-2-(4-chlorophen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from translocator protein in rat kidney mitochondrial membrane | J Med Chem 51: 5798-806 (2008) Article DOI: 10.1021/jm8003224 BindingDB Entry DOI: 10.7270/Q2GF0VD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271468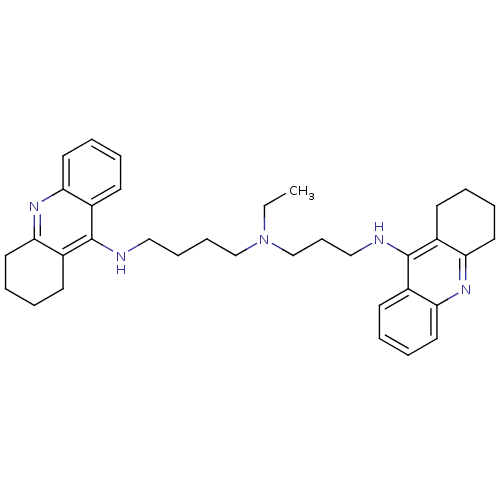 (CHEMBL490060 | N-Ethyl-N-(1,2,3,4-tetrahydroacridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50131925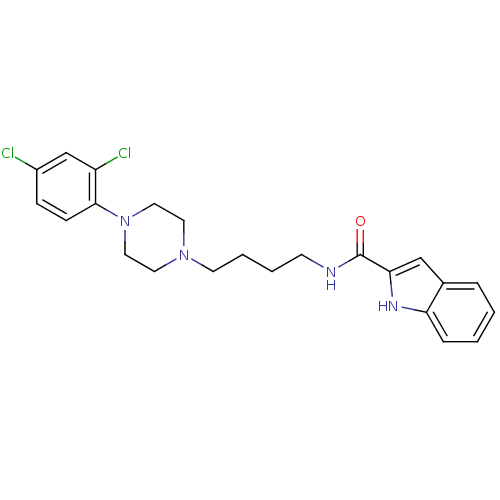 (1H-Indole-2-carboxylic acid {4-[4-(2,4-dichloro-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry | J Med Chem 52: 151-69 (2009) Article DOI: 10.1021/jm800689g BindingDB Entry DOI: 10.7270/Q2J67GSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118537 (CHEMBL135391 | Ethyl-carbamic acid 7-chloro-5-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50318267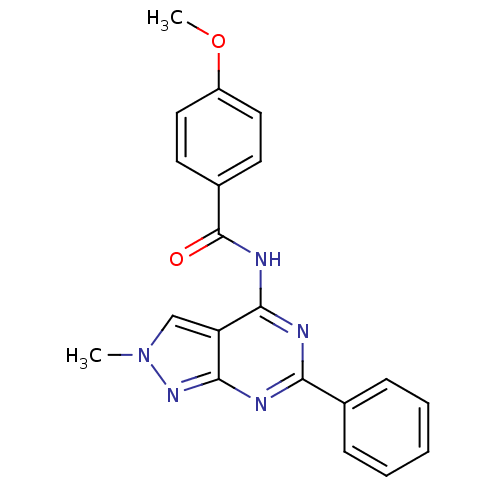 (4-methoxy-N-(2-methyl-6-phenyl-2H-pyrazolo[3,4-d]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 53: 3954-63 (2010) Article DOI: 10.1021/jm901785w BindingDB Entry DOI: 10.7270/Q2N017HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50131925 (1H-Indole-2-carboxylic acid {4-[4-(2,4-dichloro-ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Binding affinity for rat striatum Dopamine receptor D3 (sf9 cells) by [3H]7-OH-DPAT displacement. | J Med Chem 46: 3822-39 (2003) Article DOI: 10.1021/jm0211220 BindingDB Entry DOI: 10.7270/Q2H132RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50131925 (1H-Indole-2-carboxylic acid {4-[4-(2,4-dichloro-ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Displacement of [3H]-7-OH-DPAT from rat brain membrane D3 receptor expressed in Sf9 cells incubated for 60 mins by liquid scintillation counting meth... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111674 BindingDB Entry DOI: 10.7270/Q2FR012J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50253893 (2-(5-chloro-2-(4-chlorophenyl)-1H-indol-3-yl)-N-me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from translocator protein in rat kidney mitochondrial membrane | J Med Chem 51: 5798-806 (2008) Article DOI: 10.1021/jm8003224 BindingDB Entry DOI: 10.7270/Q2GF0VD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50048691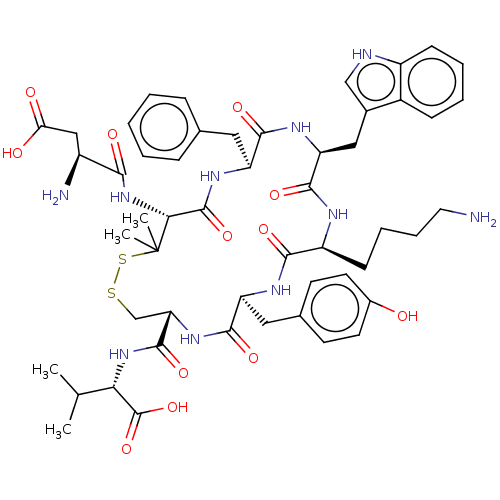 (CHEMBL3315139) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples"Federico II" Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human recombinant UT receptor expressed in CHO-K1 cells by scintillation counting method | J Med Chem 57: 5965-74 (2014) Article DOI: 10.1021/jm500218x BindingDB Entry DOI: 10.7270/Q2MC91NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413761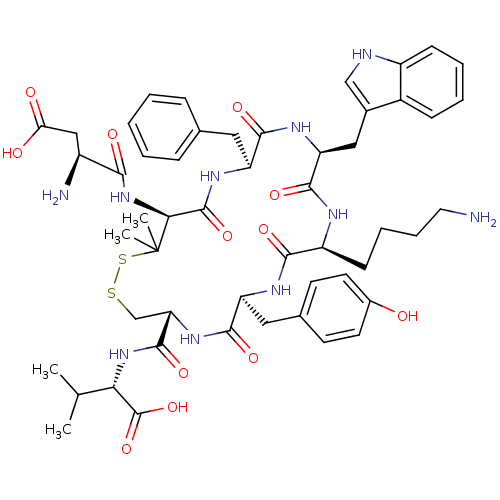 (CHEMBL390094) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno Curated by ChEMBL | Assay Description Binding affinity towards human Urotensin 2 receptor was determined | J Med Chem 47: 1652-61 (2004) Article DOI: 10.1021/jm0309912 BindingDB Entry DOI: 10.7270/Q26W9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413761 (CHEMBL390094) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Ability to displace radioligand [125I]Tyr-hU-II from human recombinant Urotensin 2 receptor in CHO-K1 cells | J Med Chem 45: 4391-4 (2002) Article DOI: 10.1021/jm025549i BindingDB Entry DOI: 10.7270/Q2FR00C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50031465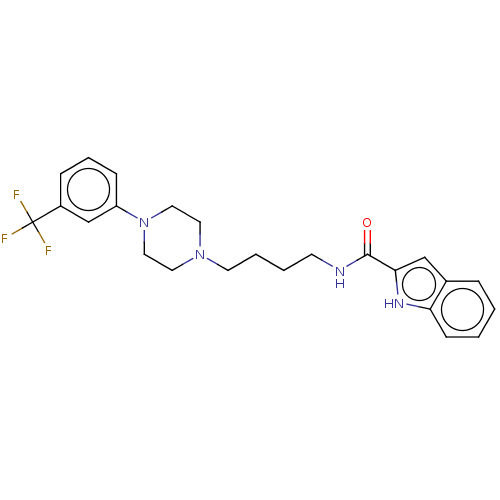 (CHEMBL3358104) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to dopamine D3 receptor (unknown origin) | J Med Chem 57: 9578-97 (2014) Article DOI: 10.1021/jm501119j BindingDB Entry DOI: 10.7270/Q2571DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413761 (CHEMBL390094) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118528 (CHEMBL135514 | Ethyl-carbamic acid 5-phenyl-6-oxa-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to mitochondrial rat testis Peripheral type benzodiazepine receptor (PBR) | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM198126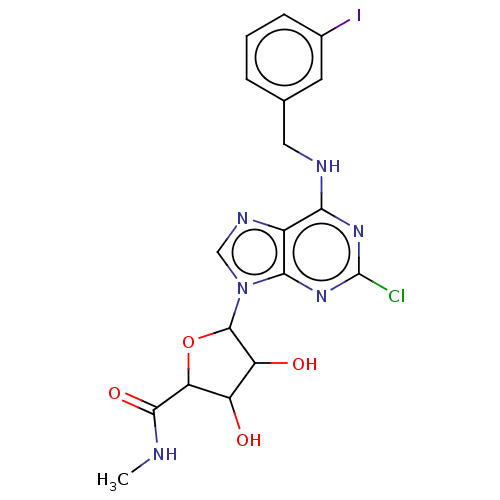 ((2S,3S,4R,5R)-5-[2-chloro-6-[(3-iodophenyl)methyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The membranes prepared from CHO cells transfected with human adenosine A3 receptors were used in binding assays. Nonspecific binding was determined i... | J Med Chem 51: 1764-70 (2008) Article DOI: 10.1021/jm701159t BindingDB Entry DOI: 10.7270/Q2MP51JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in CHO cell membrane | J Med Chem 55: 1490-9 (2012) Article DOI: 10.1021/jm201177b BindingDB Entry DOI: 10.7270/Q2CR5VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]AB-MECA from human adenosine A3 receptor expressed in CHO cells | J Med Chem 50: 5676-84 (2007) Article DOI: 10.1021/jm0708376 BindingDB Entry DOI: 10.7270/Q2SJ1MFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cell membrane after 90 mins | Eur J Med Chem 69: 331-7 (2013) Article DOI: 10.1016/j.ejmech.2013.09.001 BindingDB Entry DOI: 10.7270/Q28918TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271325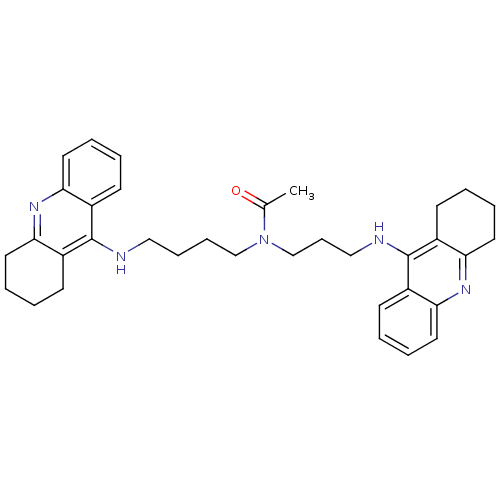 (CHEMBL451277 | N-{4-[(1,2,3,4-Tetrahydroacridin-9-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | J Med Chem 51: 3154-70 (2008) Article DOI: 10.1021/jm701253t BindingDB Entry DOI: 10.7270/Q22Z16D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8975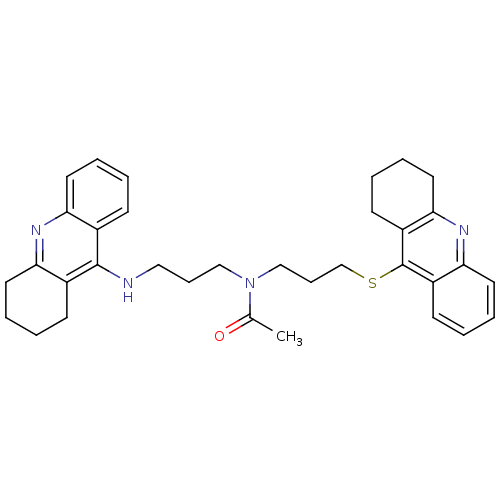 (CHEMBL179192 | N-[3-(1,2,3,4-Tetrahydroacridin-9-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita di Siena | Assay Description Inhibition of enzyme activity was measured over a substrate concentration range of 0.01-30 mM and at least six inhibitor concentrations to determine ... | J Med Chem 48: 1919-29 (2005) Article DOI: 10.1021/jm049510k BindingDB Entry DOI: 10.7270/Q27P8WMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50005192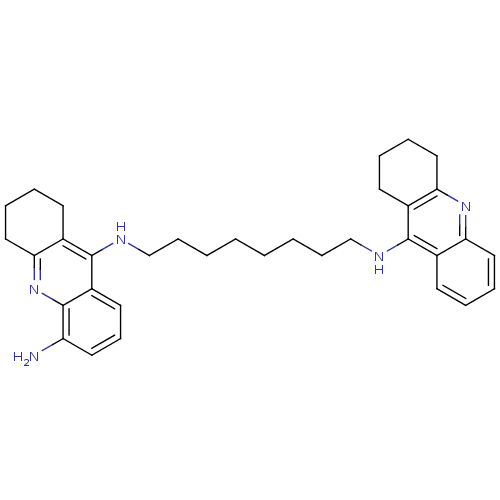 (CHEMBL3099497) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase | ACS Med Chem Lett 4: 1178-82 (2013) Article DOI: 10.1021/ml4002908 BindingDB Entry DOI: 10.7270/Q2TQ6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50253483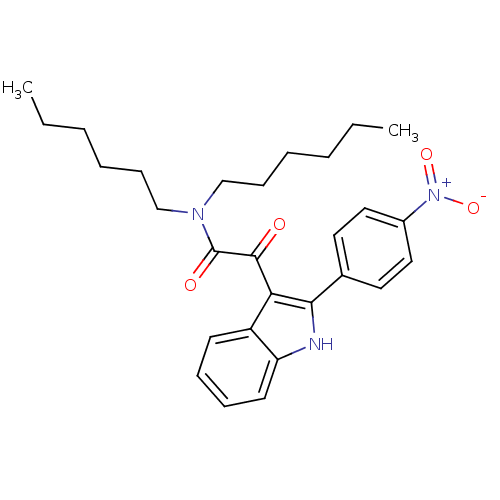 (CHEMBL492686 | N,N-dihexyl-2-(2-(4-nitrophenyl)-1H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]PK11195 from translocator protein in rat kidney mitochondrial membrane | J Med Chem 51: 5798-806 (2008) Article DOI: 10.1021/jm8003224 BindingDB Entry DOI: 10.7270/Q2GF0VD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50118539 (CHEMBL136036 | Diethyl-carbamic acid 5-thiophen-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK11195 binding to Peripheral type benzodiazepine receptor (PBR) in rat cortex homogenate by 50% | J Med Chem 45: 4276-81 (2002) BindingDB Entry DOI: 10.7270/Q20Z72MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7674 total ) | Next | Last >> |