| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tissue-type plasminogen activator |
|---|
| Ligand | BDBM50304620 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_603969 (CHEMBL1043322) |
|---|
| Ki | >60000±n/a nM |
|---|
| Citation |  Fujimoto, T; Tobisu, M; Konishi, N; Kawamura, M; Tada, N; Takagi, T; Kubo, K Synthesis and biological evaluation of the metabolites of 2-(1-{3-[(6-chloronaphthalen-2-yl)sulfonyl]propanoyl}piperidin-4-yl)-5-methyl-1,2-dihydro-3H-imidazo[1,5-c]imidazol-3-one. Bioorg Med Chem17:7993-8002 (2009) [PubMed] Article Fujimoto, T; Tobisu, M; Konishi, N; Kawamura, M; Tada, N; Takagi, T; Kubo, K Synthesis and biological evaluation of the metabolites of 2-(1-{3-[(6-chloronaphthalen-2-yl)sulfonyl]propanoyl}piperidin-4-yl)-5-methyl-1,2-dihydro-3H-imidazo[1,5-c]imidazol-3-one. Bioorg Med Chem17:7993-8002 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tissue-type plasminogen activator |
|---|
| Name: | Tissue-type plasminogen activator |
|---|
| Synonyms: | Alteplase | PLAT | Reteplase | TPA_HUMAN | Thrombin receptor protein | Tissue-type plasminogen activator | Tissue-type plasminogen activator (tPA) | Tissue-type plasminogen activator precursor | t-PA | t-Plasminogen Activator (tPA) | t-plasminogen activator |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 62931.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 562 |
|---|
| Sequence: | MDAMKRGLCCVLLLCGAVFVSPSQEIHARFRRGARSYQVICRDEKTQMIYQQHQSWLRPV
LRSNRVEYCWCNSGRAQCHSVPVKSCSEPRCFNGGTCQQALYFSDFVCQCPEGFAGKCCE
IDTRATCYEDQGISYRGTWSTAESGAECTNWNSSALAQKPYSGRRPDAIRLGLGNHNYCR
NPDRDSKPWCYVFKAGKYSSEFCSTPACSEGNSDCYFGNGSAYRGTHSLTESGASCLPWN
SMILIGKVYTAQNPSAQALGLGKHNYCRNPDGDAKPWCHVLKNRRLTWEYCDVPSCSTCG
LRQYSQPQFRIKGGLFADIASHPWQAAIFAKHRRSPGERFLCGGILISSCWILSAAHCFQ
ERFPPHHLTVILGRTYRVVPGEEEQKFEVEKYIVHKEFDDDTYDNDIALLQLKSDSSRCA
QESSVVRTVCLPPADLQLPDWTECELSGYGKHEALSPFYSERLKEAHVRLYPSSRCTSQH
LLNRTVTDNMLCAGDTRSGGPQANLHDACQGDSGGPLVCLNDGRMTLVGIISWGLGCGQK
DVPGVYTKVTNYLDWIRDNMRP
|
|
|
|---|
| BDBM50304620 |
|---|
| n/a |
|---|
| Name | BDBM50304620 |
|---|
| Synonyms: | 2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl)piperidin-4-yl)-5-(hydroxymethyl)-1H-imidazo[1,5-c]imidazol-3(2H)-one | 2-(1-{3-[(6-Chloronaphthalene-2-yl)sulfonyl]propanoyl}-piperidin-4-yl)-5-(hydroxymethyl)-1,2-dihydro-3H-imidazo[1,5-c]imidazol-3-one | CHEMBL593482 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H25ClN4O5S |
|---|
| Mol. Mass. | 516.997 |
|---|
| SMILES | OCc1ncc2CN(C3CCN(CC3)C(=O)CCS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C(=O)n12 |
|---|
| Structure | 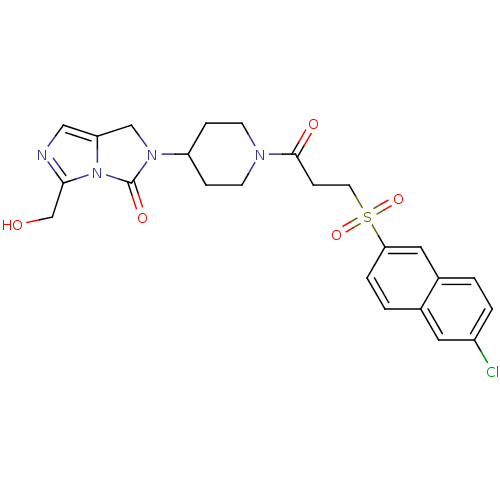
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fujimoto, T; Tobisu, M; Konishi, N; Kawamura, M; Tada, N; Takagi, T; Kubo, K Synthesis and biological evaluation of the metabolites of 2-(1-{3-[(6-chloronaphthalen-2-yl)sulfonyl]propanoyl}piperidin-4-yl)-5-methyl-1,2-dihydro-3H-imidazo[1,5-c]imidazol-3-one. Bioorg Med Chem17:7993-8002 (2009) [PubMed] Article
Fujimoto, T; Tobisu, M; Konishi, N; Kawamura, M; Tada, N; Takagi, T; Kubo, K Synthesis and biological evaluation of the metabolites of 2-(1-{3-[(6-chloronaphthalen-2-yl)sulfonyl]propanoyl}piperidin-4-yl)-5-methyl-1,2-dihydro-3H-imidazo[1,5-c]imidazol-3-one. Bioorg Med Chem17:7993-8002 (2009) [PubMed] Article