| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aromatase |
|---|
| Ligand | BDBM50322067 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_642469 (CHEMBL1175842) |
|---|
| IC50 | 1840±n/a nM |
|---|
| Citation |  Sun, B; Hoshino, J; Jermihov, K; Marler, L; Pezzuto, JM; Mesecar, AD; Cushman, M Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem18:5352-66 (2010) [PubMed] Article Sun, B; Hoshino, J; Jermihov, K; Marler, L; Pezzuto, JM; Mesecar, AD; Cushman, M Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem18:5352-66 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aromatase |
|---|
| Name: | Aromatase |
|---|
| Synonyms: | ARO1 | Aromatase | CP19A_HUMAN | CYAR | CYP19 | CYP19A1 | CYPXIX | Cytochrome P-450AROM | Cytochrome P450 19A1 | Cytochrome P450 2C19 | Cytochrome P450-C19 (CYP19) | Estrogen synthetase | FL cytokine receptor precursor | P-450AROM |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57888.92 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11511 |
|---|
| Residue: | 503 |
|---|
| Sequence: | MVLEMLNPIHYNITSIVPEAMPAATMPVLLLTGLFLLVWNYEGTSSIPGPGYCMGIGPLI
SHGRFLWMGIGSACNYYNRVYGEFMRVWISGEETLIISKSSSMFHIMKHNHYSSRFGSKL
GLQCIGMHEKGIIFNNNPELWKTTRPFFMKALSGPGLVRMVTVCAESLKTHLDRLEEVTN
ESGYVDVLTLLRRVMLDTSNTLFLRIPLDESAIVVKIQGYFDAWQALLIKPDIFFKISWL
YKKYEKSVKDLKDAIEVLIAEKRRRISTEEKLEECMDFATELILAEKRGDLTRENVNQCI
LEMLIAAPDTMSVSLFFMLFLIAKHPNVEEAIIKEIQTVIGERDIKIDDIQKLKVMENFI
YESMRYQPVVDLVMRKALEDDVIDGYPVKKGTNIILNIGRMHRLEFFPKPNEFTLENFAK
NVPYRYFQPFGFGPRGCAGKYIAMVMMKAILVTLLRRFHVKTLQGQCVESIQKIHDLSLH
PDETKNMLEMIFTPRNSDRCLEH
|
|
|
|---|
| BDBM50322067 |
|---|
| n/a |
|---|
| Name | BDBM50322067 |
|---|
| Synonyms: | 1-(4-Amino-phenyl)-2-imidazol-1-yl-ethanone | 1-(4-Aminophenyl)-2-(1H-imidazol-1-yl)ethanone | CHEMBL162431 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C11H11N3O |
|---|
| Mol. Mass. | 201.2245 |
|---|
| SMILES | Nc1ccc(cc1)C(=O)Cn1ccnc1 |
|---|
| Structure | 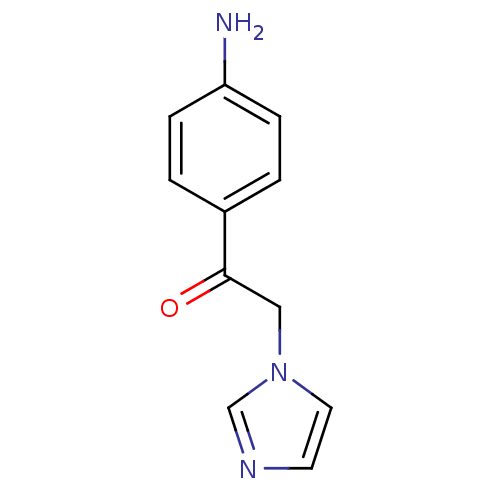
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sun, B; Hoshino, J; Jermihov, K; Marler, L; Pezzuto, JM; Mesecar, AD; Cushman, M Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem18:5352-66 (2010) [PubMed] Article
Sun, B; Hoshino, J; Jermihov, K; Marler, L; Pezzuto, JM; Mesecar, AD; Cushman, M Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem18:5352-66 (2010) [PubMed] Article