

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983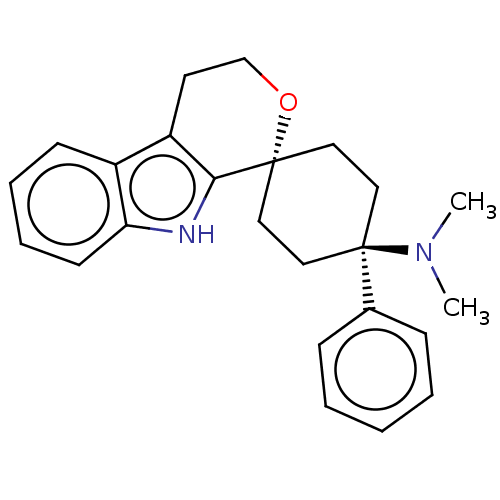 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127310 (US8791268, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101099 (CHEMBL3326228) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China State Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5HT1A receptor (unknown origin) expressed in HEK293 cells membranes incubated for 60 mins by scintillation counti... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126703 BindingDB Entry DOI: 10.7270/Q2DR2ZZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101306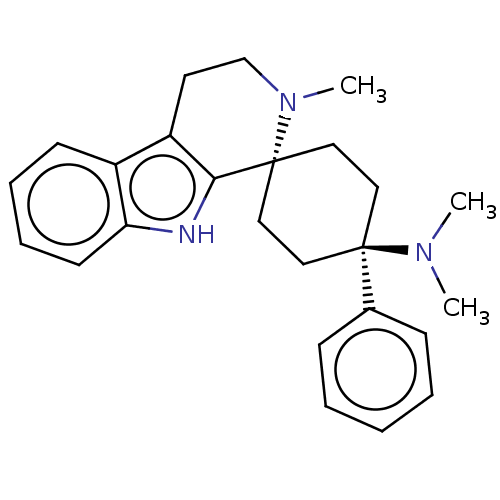 (CHEMBL3326229) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177911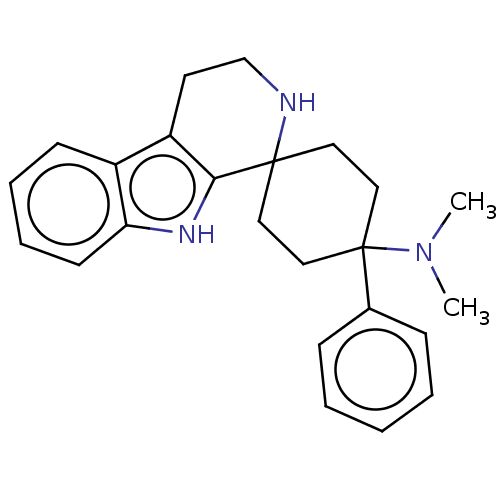 (US9120797, 10 | US9120797, 9) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127312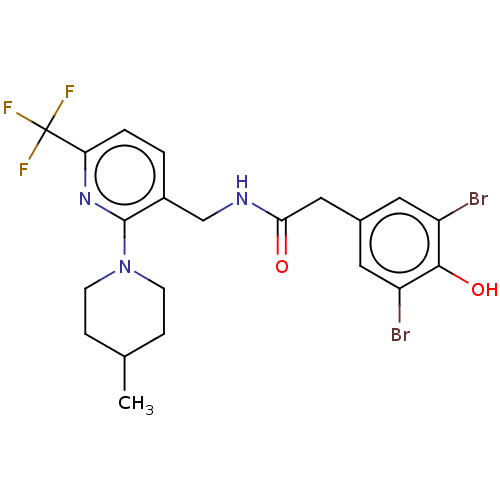 (US8791268, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101088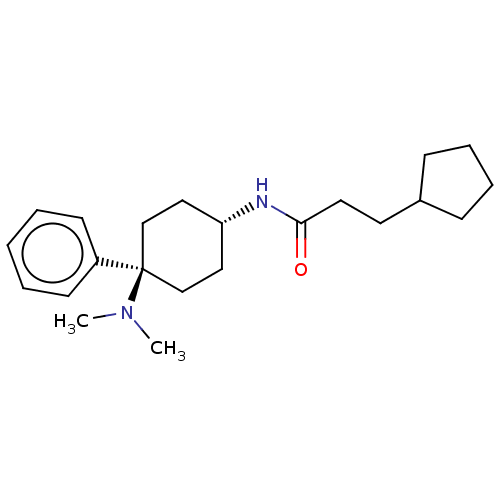 (CHEMBL3326220) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101152 (CHEMBL3326232) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177911 (US9120797, 10 | US9120797, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | -53.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101099 (CHEMBL3326228) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101091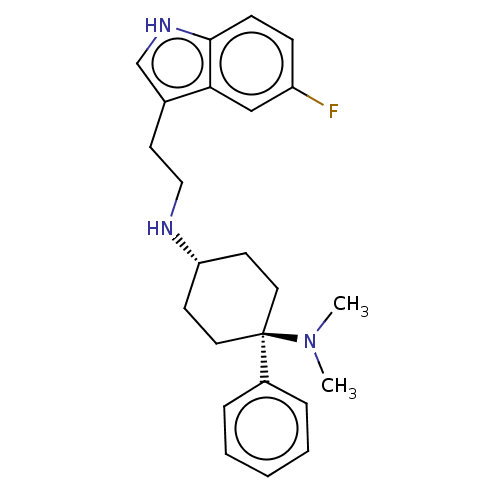 (CHEMBL3326223) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101096 (CHEMBL3325961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177935 (US9120797, 33) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101088 (CHEMBL3326220) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100991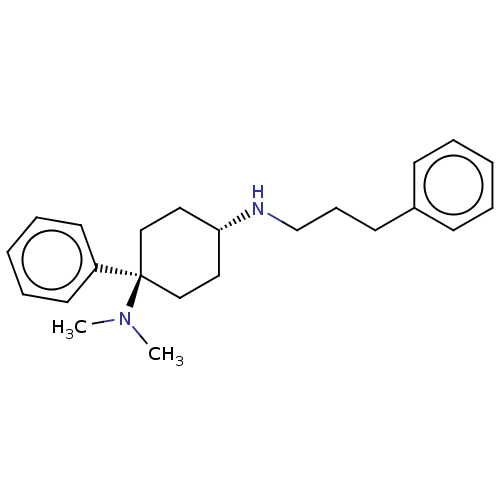 (CHEMBL3325879) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101306 (CHEMBL3326229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177955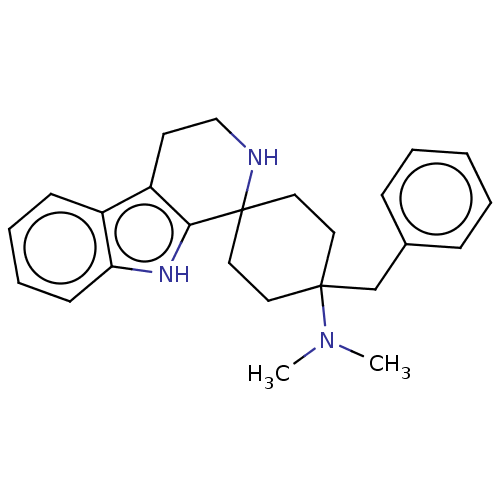 (US9120797, 53) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100993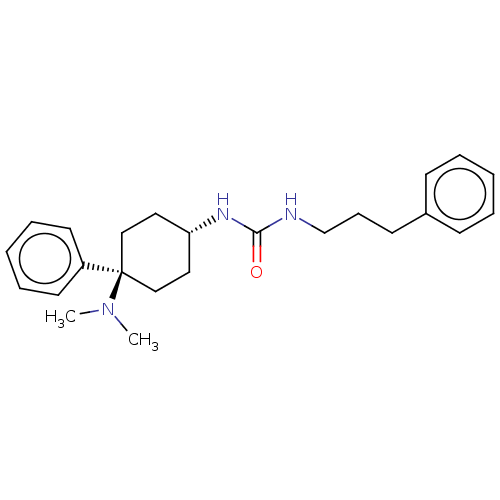 (CHEMBL3325881) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177936 (US9120797, 34) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101095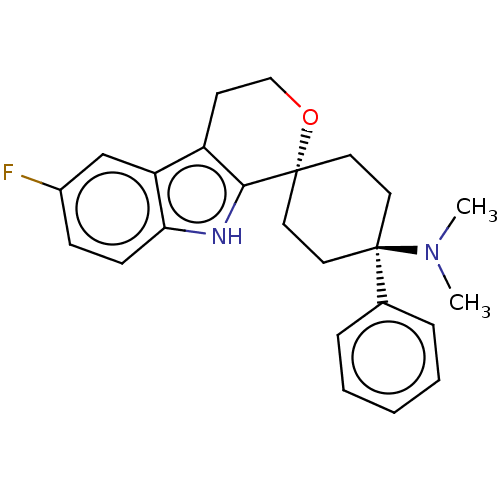 (CHEMBL3325957) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50088373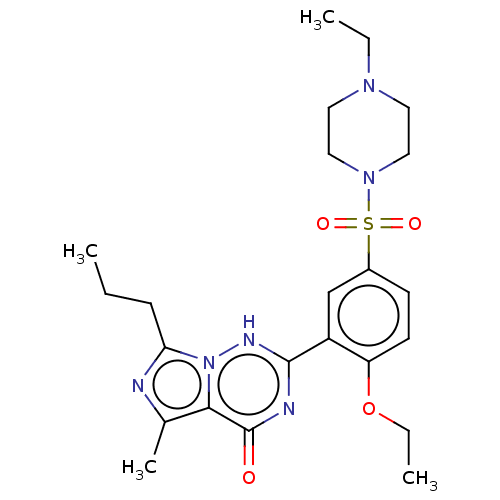 (CHEBI:46295 | Vardenafil | cid_110634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of PDE5 (unknown origin) | Eur J Med Chem 158: 767-780 (2018) Article DOI: 10.1016/j.ejmech.2018.09.028 BindingDB Entry DOI: 10.7270/Q2JS9T4N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127311 (US8791268, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50433561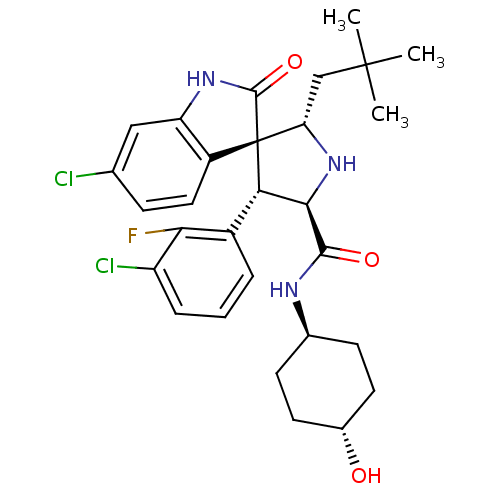 (CHEMBL2381408) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant human His6-tagged HDM2 (1 to 118 residues) assessed as reduction in PMDM6-F binding incubated for 15 to 30 mins by fl... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01524 BindingDB Entry DOI: 10.7270/Q2Z03D1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101095 (CHEMBL3325957) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50529075 (CHEMBL4544153) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China State Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from 5HT7 receptor (unknown origin) expressed in CHO cell membranes incubated for 120 mins by scintillation counting method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126703 BindingDB Entry DOI: 10.7270/Q2DR2ZZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177948 (US9120797, 46) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127309 (US8791268, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50529074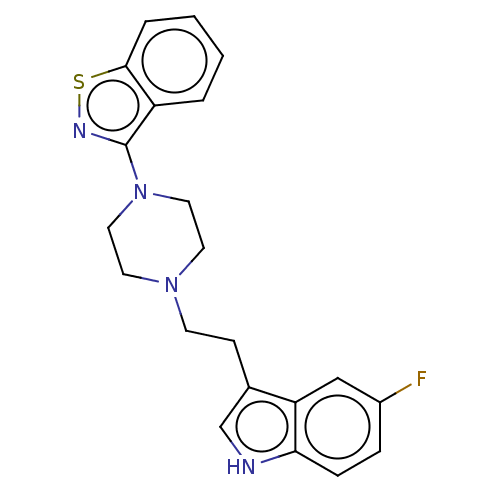 (CHEMBL4574299) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China State Institute of Pharmaceutical Industry Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from 5HT7 receptor (unknown origin) expressed in CHO cell membranes incubated for 120 mins by scintillation counting method | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126703 BindingDB Entry DOI: 10.7270/Q2DR2ZZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101096 (CHEMBL3325961) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50350022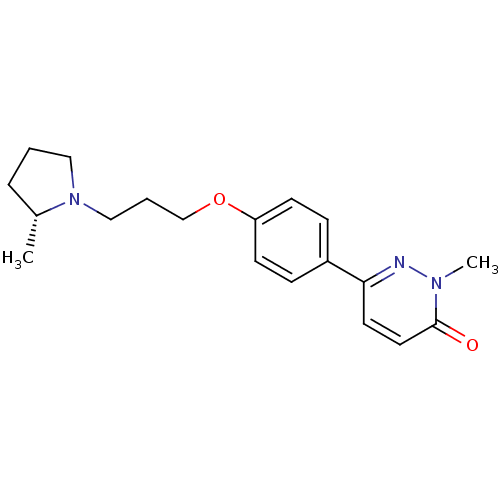 (CHEMBL1813057) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NAMH from human histamine H3 receptor | Bioorg Med Chem Lett 21: 5543-6 (2011) Article DOI: 10.1016/j.bmcl.2011.06.094 BindingDB Entry DOI: 10.7270/Q2QF8TW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101100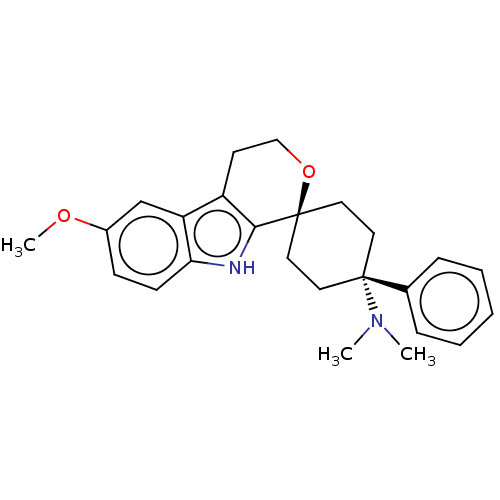 (CHEMBL3325962) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101090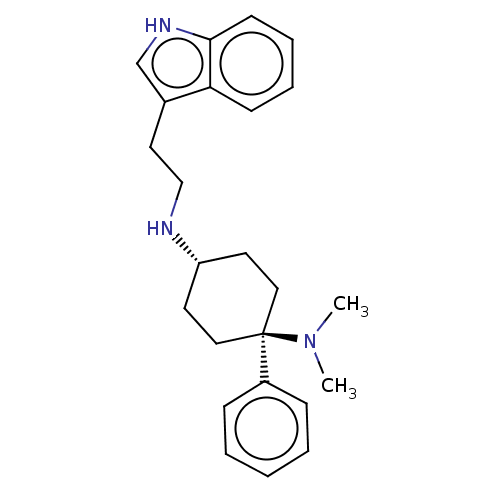 (CHEMBL3326222) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177934 (US9120797, 32) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177955 (US9120797, 53) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127308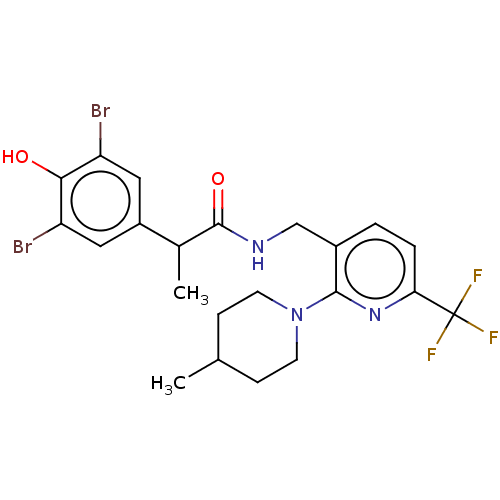 (US8791268, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101152 (CHEMBL3326232) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177947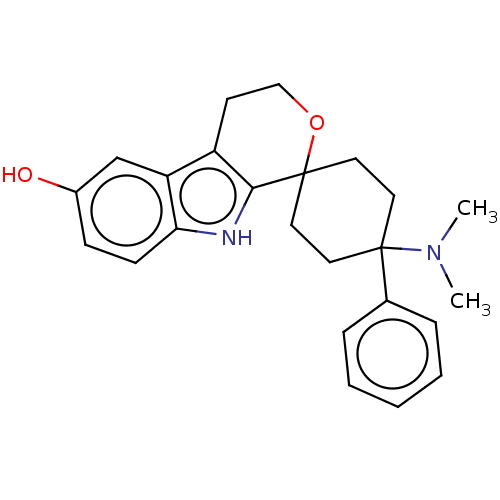 (US9120797, 45) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177925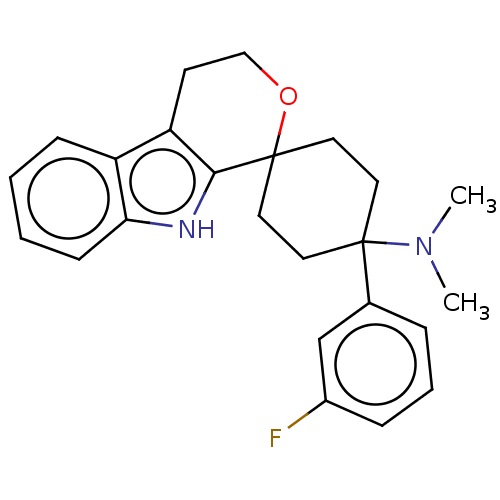 (US9120797, 23) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM177916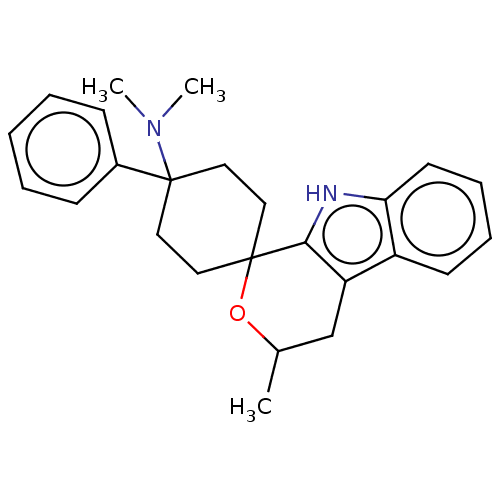 (US9120797, 14 | US9120797, 15) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Gruenenthal GmbH US Patent | Assay Description The cyclohexane derivatives of the general formula I were investigated in a receptor binding assay with 3H-nociceptin/orphanin FQ with membranes from... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177930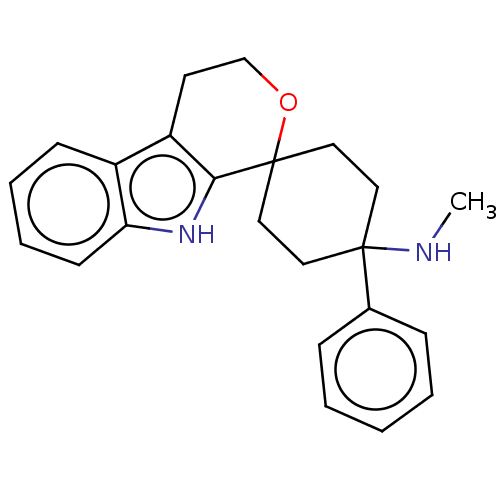 (US9120797, 28) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM177903 (US9120797, 1 | US9120797, 2 | US9120797, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Gruenenthal GmbH US Patent | Assay Description The receptor affinity for the human mu-opiate receptor was determined in a homogeneous batch in microtitre plates. For this, dilution series of the p... | US Patent US9120797 (2015) BindingDB Entry DOI: 10.7270/Q2X065T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101157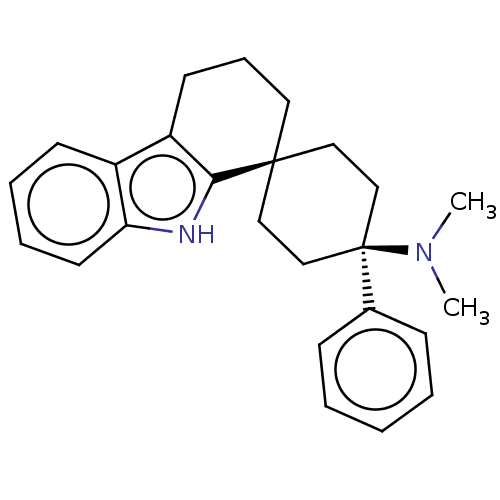 (CHEMBL3326231) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4294 total ) | Next | Last >> |