

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366125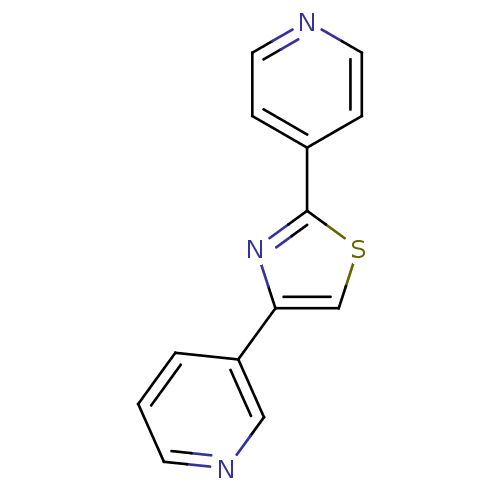 (CHEMBL1957214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015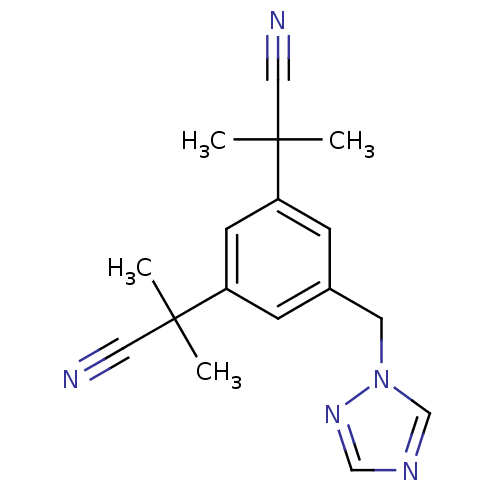 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366128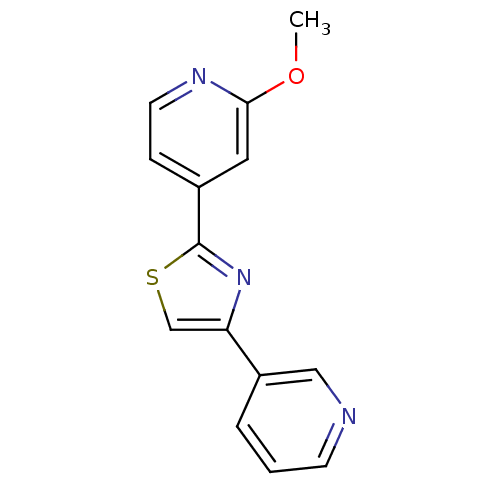 (CHEMBL1957217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366129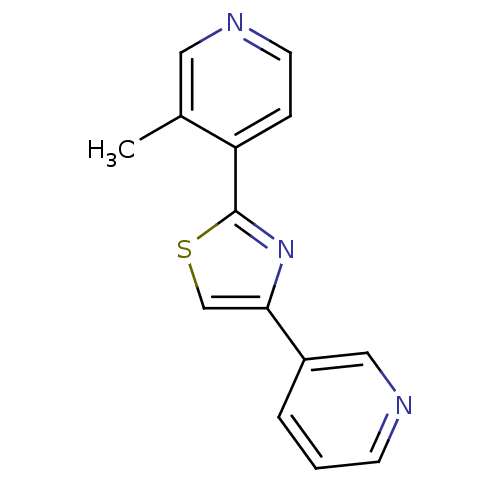 (CHEMBL1957218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50102258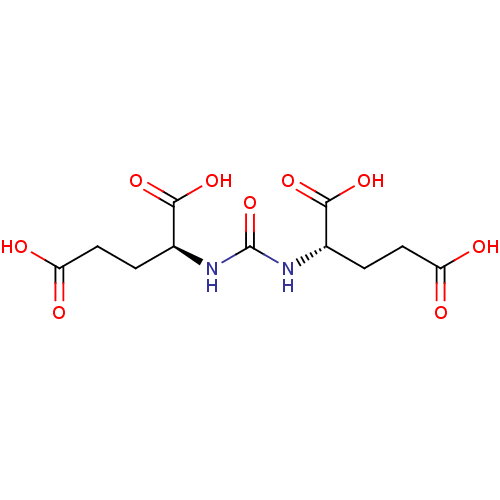 ((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity PSMA (unknown origin) | J Med Chem 58: 3094-103 (2015) Article DOI: 10.1021/jm5018384 BindingDB Entry DOI: 10.7270/Q2MG7R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366127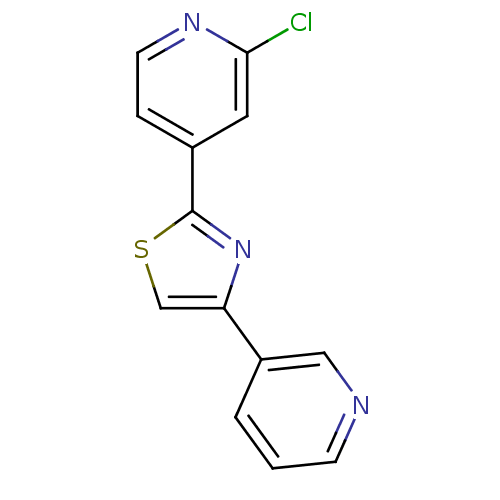 (CHEMBL1957216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366134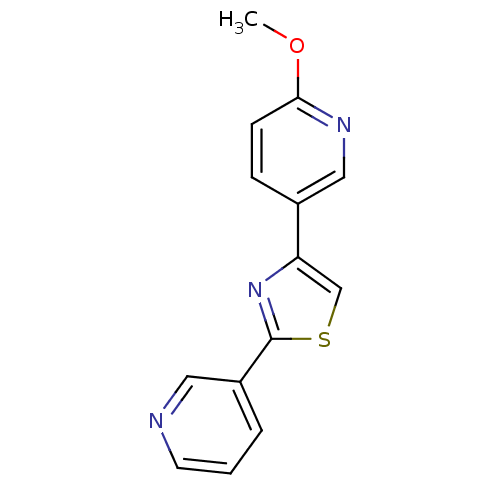 (CHEMBL1957223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal aromatase using 7-methoxy-4-trifluoromethylcoumarin as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 4611-8 (2013) Article DOI: 10.1021/jm400364h BindingDB Entry DOI: 10.7270/Q2TX3GR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 assessed as metabolism of 3-cyano-7-ethoxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366120 (CHEMBL1601919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 assessed as metabolism of 3-cyano-7-ethoxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366132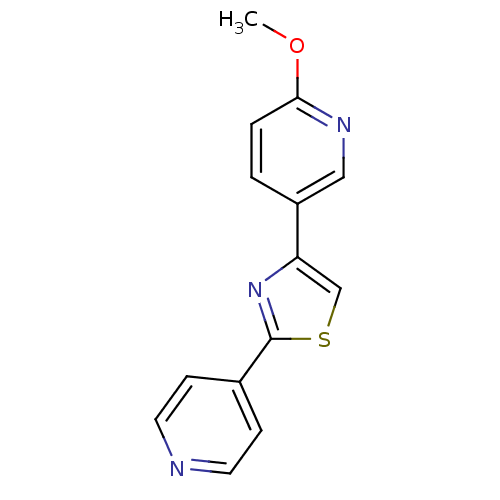 (CHEMBL1957221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366133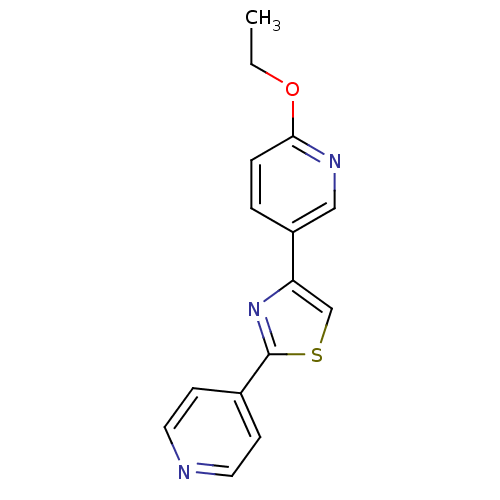 (CHEMBL1957222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366122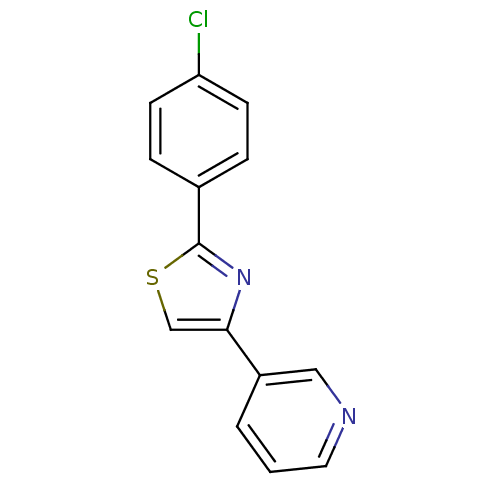 (CHEMBL1957211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366130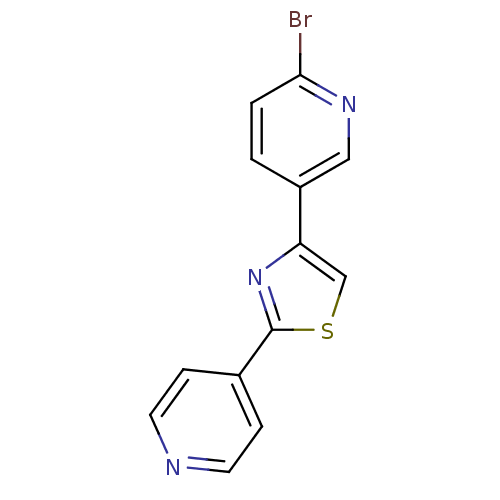 (CHEMBL1957219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 423 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435004 (CHEMBL2386284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 442 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal aromatase using 7-methoxy-4-trifluoromethylcoumarin as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 4611-8 (2013) Article DOI: 10.1021/jm400364h BindingDB Entry DOI: 10.7270/Q2TX3GR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435004 (CHEMBL2386284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 442 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal aromatase using 7-methoxy-4-trifluoromethylcoumarin as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 4611-8 (2013) Article DOI: 10.1021/jm400364h BindingDB Entry DOI: 10.7270/Q2TX3GR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2A6 assessed as metabolism of coumarin to 7-hydroxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 855 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A5 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366118 (CHEMBL1957208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366131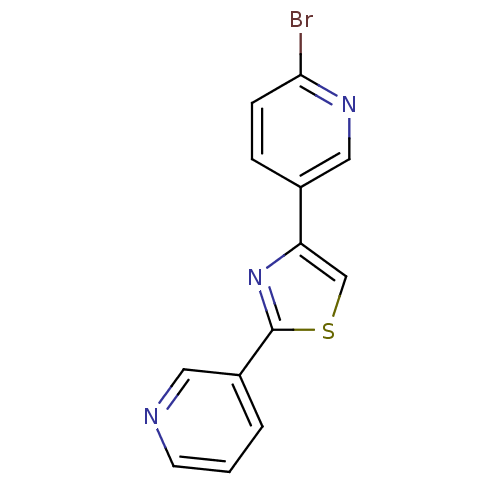 (CHEMBL1957220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50081468 (CHEMBL3422028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 assessed as metabolism of 7-benzyloxy-4-trifluoromethylcoumarin to HFC after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366123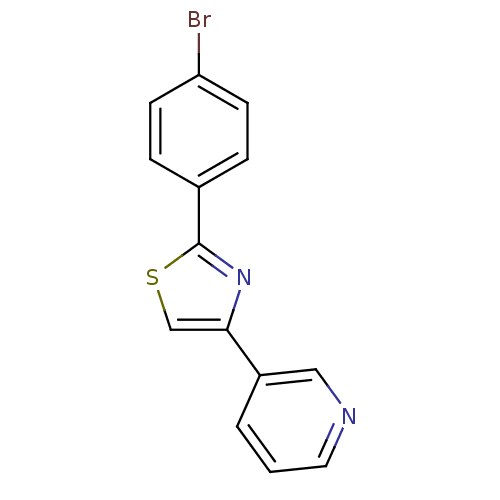 (CHEMBL1957212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50435005 (CHEMBL2386285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2A6 assessed as metabolism of coumarin to 7-hydroxycoumarin after 30 mins by fluorescence assay | J Med Chem 58: 2623-48 (2015) Article DOI: 10.1021/jm501218e BindingDB Entry DOI: 10.7270/Q2TX3H32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366126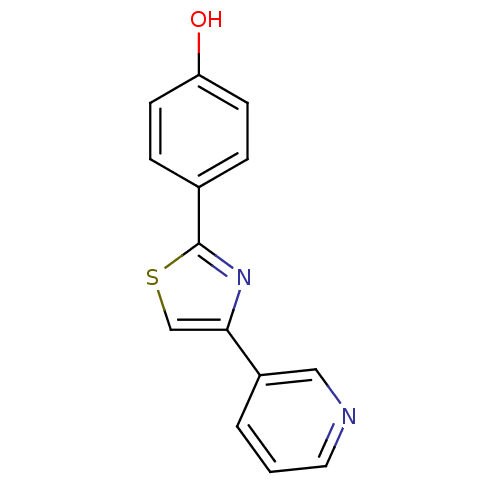 (CHEMBL1957215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 1 (Homo sapiens (Human)) | BDBM50388546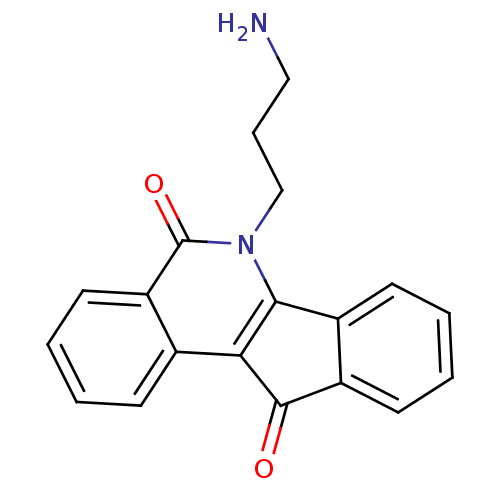 (CHEMBL213072 | CHEMBL333363) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant Tdp1 after 1 hr by FRET assay | J Med Chem 55: 4457-78 (2012) Article DOI: 10.1021/jm300335n BindingDB Entry DOI: 10.7270/Q2SB46TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366119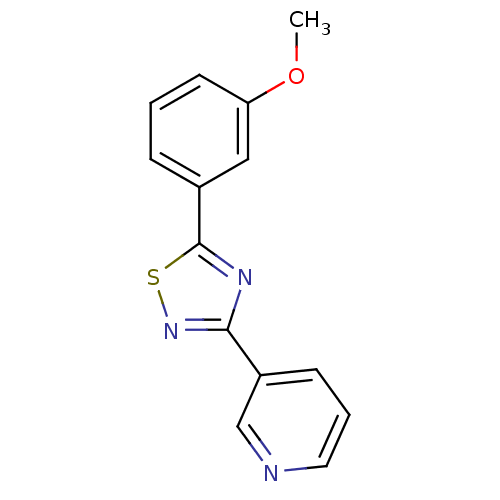 (CHEMBL1957209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316579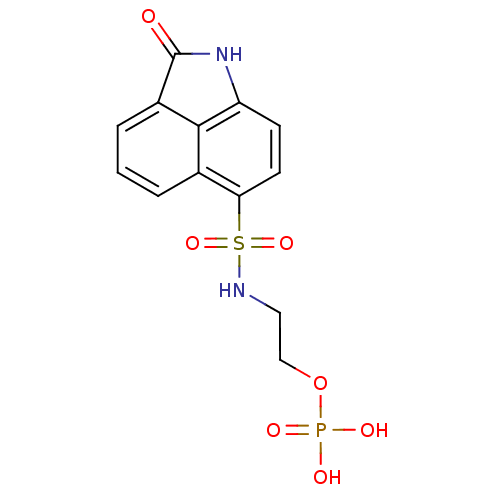 (2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316577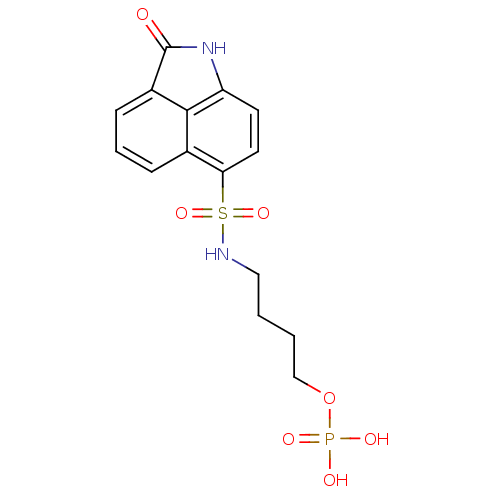 (4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366121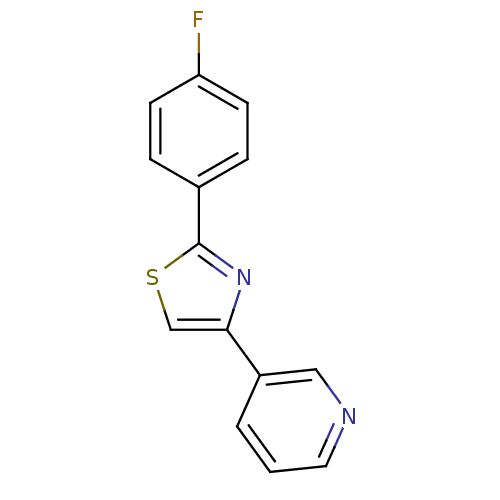 (CHEMBL1957210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316578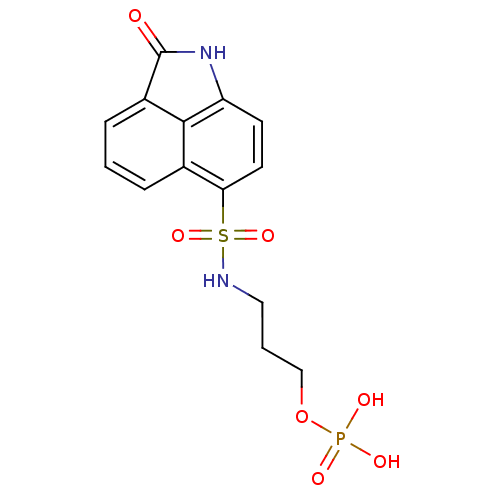 (3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50366124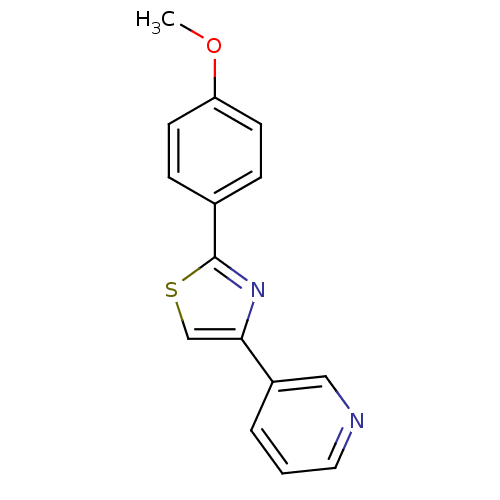 (CHEMBL1957213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316576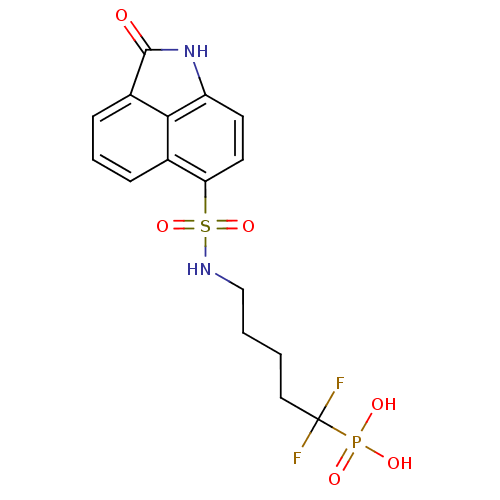 (1,1-difluoro-5-(2-oxo-1,2-dihydrobenzo[cd]indole-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316580 (6-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316581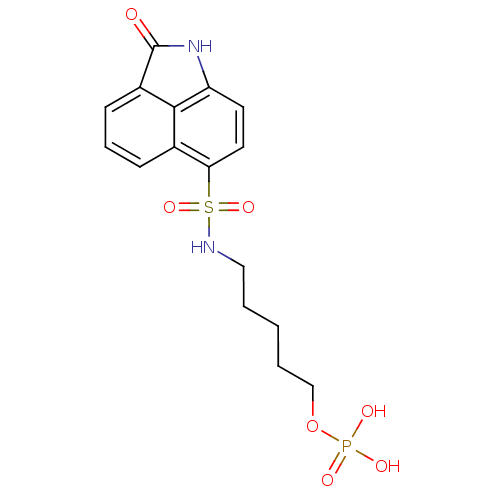 (5-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316575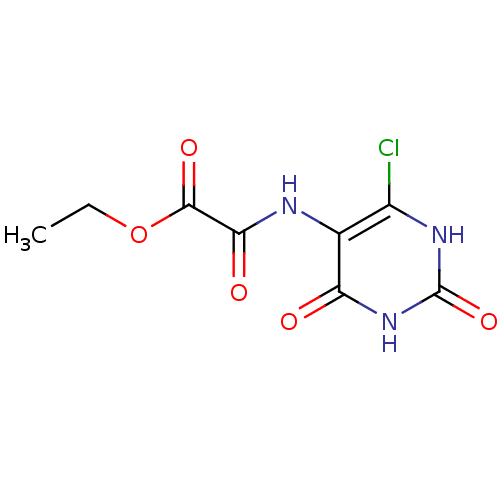 (CHEMBL1095229 | Ethyl2-(6-chloro-2,4,dioxo-1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316577 (4-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... | Bioorg Med Chem 20: 2427-34 (2012) Article DOI: 10.1016/j.bmc.2012.01.047 BindingDB Entry DOI: 10.7270/Q2DJ5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316574 (2-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316574 (2-(2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316578 (3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316579 (2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316579 (2-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-inhibitor complex state after 30 mins by fluorescence assay | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316578 (3-(2-Oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6,7-dimethyl-8-ribityllumazine synthase (Mycobacterium tuberculosis) | BDBM50316576 (1,1-difluoro-5-(2-oxo-1,2-dihydrobenzo[cd]indole-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant lumazine synthase at enzyme-substrate-inhibitor complex state after 30 mins by fluorescence assa... | Bioorg Med Chem 18: 3518-34 (2010) Article DOI: 10.1016/j.bmc.2010.03.072 BindingDB Entry DOI: 10.7270/Q29P31S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1671 total ) | Next | Last >> |