

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50102258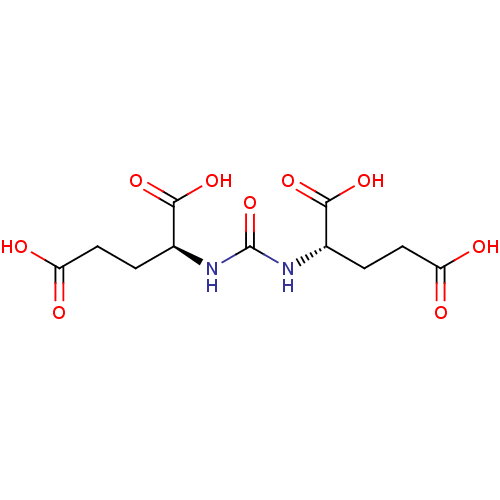 ((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity PSMA (unknown origin) | J Med Chem 58: 3094-103 (2015) Article DOI: 10.1021/jm5018384 BindingDB Entry DOI: 10.7270/Q2MG7R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50102258 ((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCPII expressed in CHO cells assessed as accumulation of glutamate using NAAG as substrate by e Amplex Red glutamic acid assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128044 BindingDB Entry DOI: 10.7270/Q2SQ9459 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50102258 ((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Binding affinity to NAALADase | J Med Chem 55: 9510-20 (2012) Article DOI: 10.1021/jm300710j BindingDB Entry DOI: 10.7270/Q28053R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50102258 ((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) | J Med Chem 58: 3094-103 (2015) Article DOI: 10.1021/jm5018384 BindingDB Entry DOI: 10.7270/Q2MG7R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50102258 ((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against expressed rat Glutamate carboxypeptidase II, using a substrate concentration of 5 microM | J Med Chem 44: 298-301 (2001) BindingDB Entry DOI: 10.7270/Q2KD1X7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50102258 ((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GCPII (unknown origin) expressed in DU-145 cells after 2 hrs by Amplex red reagent based fluorescence assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128044 BindingDB Entry DOI: 10.7270/Q2SQ9459 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||