| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine/threonine-protein kinase PLK3 |
|---|
| Ligand | BDBM50329935 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_675879 (CHEMBL1272696) |
|---|
| IC50 | 485±n/a nM |
|---|
| Citation |  Beria, I; Valsasina, B; Brasca, MG; Ceccarelli, W; Colombo, M; Cribioli, S; Fachin, G; Ferguson, RD; Fiorentini, F; Gianellini, LM; Giorgini, ML; Moll, JK; Posteri, H; Pezzetta, D; Roletto, F; Sola, F; Tesei, D; Caruso, M 4,5-Dihydro-1H-pyrazolo[4,3-h]quinazolines as potent and selective Polo-like kinase 1 (PLK1) inhibitors. Bioorg Med Chem Lett20:6489-94 (2010) [PubMed] Article Beria, I; Valsasina, B; Brasca, MG; Ceccarelli, W; Colombo, M; Cribioli, S; Fachin, G; Ferguson, RD; Fiorentini, F; Gianellini, LM; Giorgini, ML; Moll, JK; Posteri, H; Pezzetta, D; Roletto, F; Sola, F; Tesei, D; Caruso, M 4,5-Dihydro-1H-pyrazolo[4,3-h]quinazolines as potent and selective Polo-like kinase 1 (PLK1) inhibitors. Bioorg Med Chem Lett20:6489-94 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Serine/threonine-protein kinase PLK3 |
|---|
| Name: | Serine/threonine-protein kinase PLK3 |
|---|
| Synonyms: | CNK | Cytokine-inducible serine/threonine-protein kinase | FGF-inducible kinase | FNK | PLK3 | PLK3_HUMAN | PRK | Polo-Like Kinase 3 | Proliferation-related kinase | Serine/threonine-protein kinase PLK3 |
|---|
| Type: | Serine/threonine-protein kinase |
|---|
| Mol. Mass.: | 71655.17 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Enzyme assays were using PLK3 purified from baculovirus-infected Trichoplusia ni cells expressing full-length PLK3. |
|---|
| Residue: | 646 |
|---|
| Sequence: | MEPAAGFLSPRPFQRAAAAPAPPAGPGPPPSALRGPELEMLAGLPTSDPGRLITDPRSGR
TYLKGRLLGKGGFARCYEATDTETGSAYAVKVIPQSRVAKPHQREKILNEIELHRDLQHR
HIVRFSHHFEDADNIYIFLELCSRKSLAHIWKARHTLLEPEVRYYLRQILSGLKYLHQRG
ILHRDLKLGNFFITENMELKVGDFGLAARLEPPEQRKKTICGTPNYVAPEVLLRQGHGPE
ADVWSLGCVMYTLLCGSPPFETADLKETYRCIKQVHYTLPASLSLPARQLLAAILRASPR
DRPSIDQILRHDFFTKGYTPDRLPISSCVTVPDLTPPNPARSLFAKVTKSLFGRKKKSKN
HAQERDEVSGLVSGLMRTSVGHQDARPEAPAASGPAPVSLVETAPEDSSPRGTLASSGDG
FEEGLTVATVVESALCALRNCIAFMPPAEQNPAPLAQPEPLVWVSKWVDYSNKFGFGYQL
SSRRVAVLFNDGTHMALSANRKTVHYNPTSTKHFSFSVGAVPRALQPQLGILRYFASYME
QHLMKGGDLPSVEEVEVPAPPLLLQWVKTDQALLMLFSDGTVQVNFYGDHTKLILSGWEP
LLVTFVARNRSACTYLASHLRQLGCSPDLRQRLRYALRLLRDRSPA
|
|
|
|---|
| BDBM50329935 |
|---|
| n/a |
|---|
| Name | BDBM50329935 |
|---|
| Synonyms: | 1-methyl-8-(5-(2-(1-methylpyrrolidin-2-yl)ethylamino)-2-(trifluoromethoxy)phenylamino)-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide | CHEMBL1271535 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H29F3N8O2 |
|---|
| Mol. Mass. | 530.5454 |
|---|
| SMILES | CN1CCCC1CCNc1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 |
|---|
| Structure | 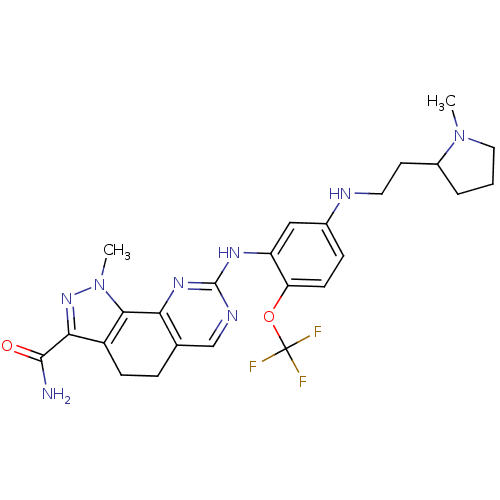
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Beria, I; Valsasina, B; Brasca, MG; Ceccarelli, W; Colombo, M; Cribioli, S; Fachin, G; Ferguson, RD; Fiorentini, F; Gianellini, LM; Giorgini, ML; Moll, JK; Posteri, H; Pezzetta, D; Roletto, F; Sola, F; Tesei, D; Caruso, M 4,5-Dihydro-1H-pyrazolo[4,3-h]quinazolines as potent and selective Polo-like kinase 1 (PLK1) inhibitors. Bioorg Med Chem Lett20:6489-94 (2010) [PubMed] Article
Beria, I; Valsasina, B; Brasca, MG; Ceccarelli, W; Colombo, M; Cribioli, S; Fachin, G; Ferguson, RD; Fiorentini, F; Gianellini, LM; Giorgini, ML; Moll, JK; Posteri, H; Pezzetta, D; Roletto, F; Sola, F; Tesei, D; Caruso, M 4,5-Dihydro-1H-pyrazolo[4,3-h]quinazolines as potent and selective Polo-like kinase 1 (PLK1) inhibitors. Bioorg Med Chem Lett20:6489-94 (2010) [PubMed] Article