 Found 280 hits with Last Name = 'giorgini' and Initial = 'ml'
Found 280 hits with Last Name = 'giorgini' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329915

(1-methyl-8-(5-(piperazin-1-yl)-2-(trifluoromethoxy...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4cc(ccc4OC(F)(F)F)N4CCNCC4)nc3-c12 Show InChI InChI=1S/C22H23F3N8O2/c1-32-19-14(18(31-32)20(26)34)4-2-12-11-28-21(30-17(12)19)29-15-10-13(33-8-6-27-7-9-33)3-5-16(15)35-22(23,24)25/h3,5,10-11,27H,2,4,6-9H2,1H3,(H2,26,34)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329914
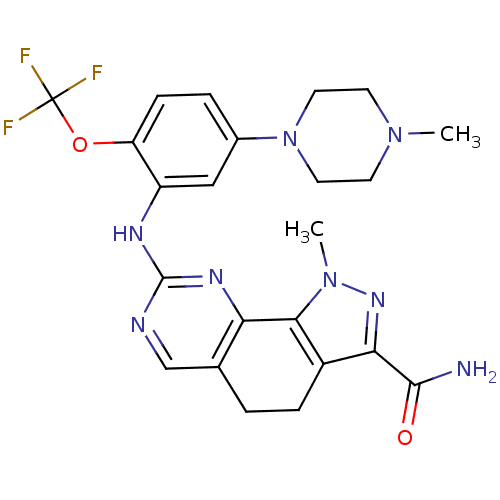
(1-methyl-8-(5-(4-methylpiperazin-1-yl)-2-(trifluor...)Show SMILES CN1CCN(CC1)c1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C23H25F3N8O2/c1-32-7-9-34(10-8-32)14-4-6-17(36-23(24,25)26)16(11-14)29-22-28-12-13-3-5-15-19(21(27)35)31-33(2)20(15)18(13)30-22/h4,6,11-12H,3,5,7-10H2,1-2H3,(H2,27,35)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329913
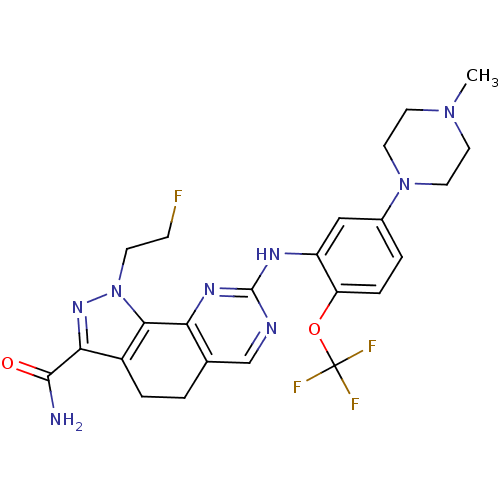
(1-(2-fluoroethyl)-8-(5-(piperazin-1-yl)-2-(trifluo...)Show SMILES CN1CCN(CC1)c1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(nn(CCF)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C24H26F4N8O2/c1-34-8-10-35(11-9-34)15-3-5-18(38-24(26,27)28)17(12-15)31-23-30-13-14-2-4-16-20(22(29)37)33-36(7-6-25)21(16)19(14)32-23/h3,5,12-13H,2,4,6-11H2,1H3,(H2,29,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329926

(8-(5-(piperazin-1-yl)-2-(trifluoromethoxy)phenylam...)Show SMILES CN1CCN(CC1)c1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(n[nH]c4C(N)=O)-c3n2)c1 Show InChI InChI=1S/C22H23F3N8O2/c1-32-6-8-33(9-7-32)13-3-5-16(35-22(23,24)25)15(10-13)28-21-27-11-12-2-4-14-18(17(12)29-21)30-31-19(14)20(26)34/h3,5,10-11H,2,4,6-9H2,1H3,(H2,26,34)(H,30,31)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12103
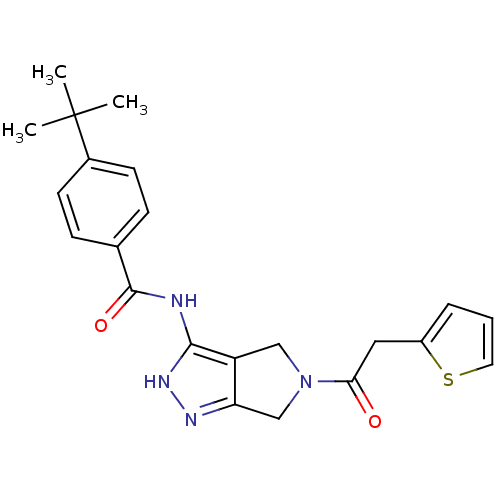
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 11 | 4-te...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1cccs1 Show InChI InChI=1S/C22H24N4O2S/c1-22(2,3)15-8-6-14(7-9-15)21(28)23-20-17-12-26(13-18(17)24-25-20)19(27)11-16-5-4-10-29-16/h4-10H,11-13H2,1-3H3,(H2,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327930

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O3S/c1-16(17-5-3-2-4-6-17)26-25(32)21-15-20-22(34-21)23(29-28-20)27-24(31)18-7-9-19(10-8-18)30-11-13-33-14-12-30/h2-10,15-16H,11-14H2,1H3,(H,26,32)(H2,27,28,29,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329927

(1-ethyl-8-(5-(piperazin-1-yl)-2-(trifluoromethoxy)...)Show SMILES CCn1nc(C(N)=O)c2CCc3cnc(Nc4cc(ccc4OC(F)(F)F)N4CCN(C)CC4)nc3-c12 Show InChI InChI=1S/C24H27F3N8O2/c1-3-35-21-16(20(32-35)22(28)36)6-4-14-13-29-23(31-19(14)21)30-17-12-15(34-10-8-33(2)9-11-34)5-7-18(17)37-24(25,26)27/h5,7,12-13H,3-4,6,8-11H2,1-2H3,(H2,28,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327929
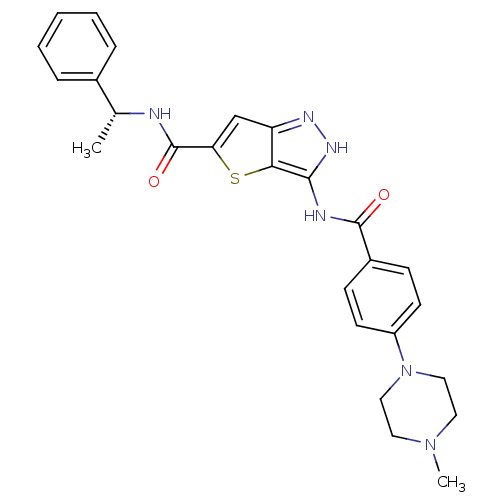
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12983
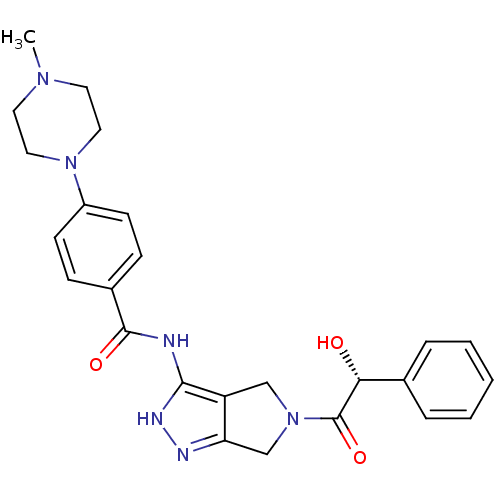
(5-Amido-pyrrolopyrazole 9b | CHEMBL385872 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C25H28N6O3/c1-29-11-13-30(14-12-29)19-9-7-18(8-10-19)24(33)26-23-20-15-31(16-21(20)27-28-23)25(34)22(32)17-5-3-2-4-6-17/h2-10,22,32H,11-16H2,1H3,(H2,26,27,28,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329929
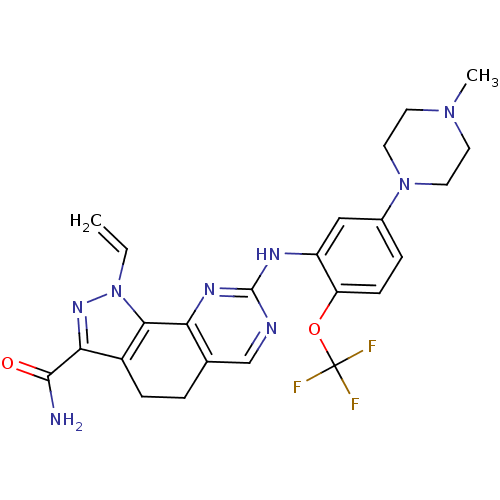
(8-(5-(piperazin-1-yl)-2-(trifluoromethoxy)phenylam...)Show SMILES CN1CCN(CC1)c1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(nn(C=C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C24H25F3N8O2/c1-3-35-21-16(20(32-35)22(28)36)6-4-14-13-29-23(31-19(14)21)30-17-12-15(34-10-8-33(2)9-11-34)5-7-18(17)37-24(25,26)27/h3,5,7,12-13H,1,4,6,8-11H2,2H3,(H2,28,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329916

(1-(2-chloroethyl)-8-(5-(piperazin-1-yl)-2-(trifluo...)Show SMILES CN1CCN(CC1)c1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(nn(CCCl)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C24H26ClF3N8O2/c1-34-8-10-35(11-9-34)15-3-5-18(38-24(26,27)28)17(12-15)31-23-30-13-14-2-4-16-20(22(29)37)33-36(7-6-25)21(16)19(14)32-23/h3,5,12-13H,2,4,6-11H2,1H3,(H2,29,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329921

(8-(5-(4-ethylpiperazin-1-yl)-2-(trifluoromethoxy)p...)Show SMILES CCN1CCN(CC1)c1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C24H27F3N8O2/c1-3-34-8-10-35(11-9-34)15-5-7-18(37-24(25,26)27)17(12-15)30-23-29-13-14-4-6-16-20(22(28)36)32-33(2)21(16)19(14)31-23/h5,7,12-13H,3-4,6,8-11H2,1-2H3,(H2,28,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12982
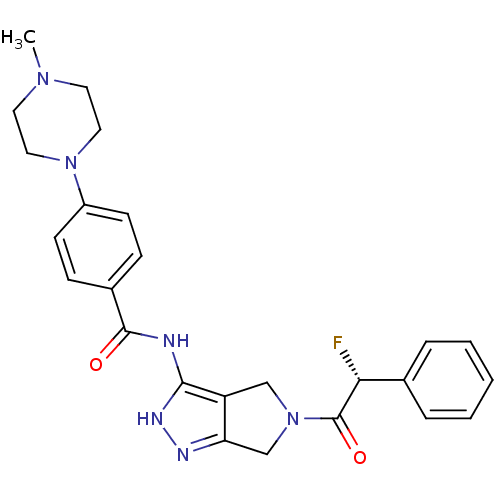
(5-Amido-pyrrolopyrazole 9a | CHEMBL385266 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](F)c1ccccc1 |r| Show InChI InChI=1S/C25H27FN6O2/c1-30-11-13-31(14-12-30)19-9-7-18(8-10-19)24(33)27-23-20-15-32(16-21(20)28-29-23)25(34)22(26)17-5-3-2-4-6-17/h2-10,22H,11-16H2,1H3,(H2,27,28,29,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327928
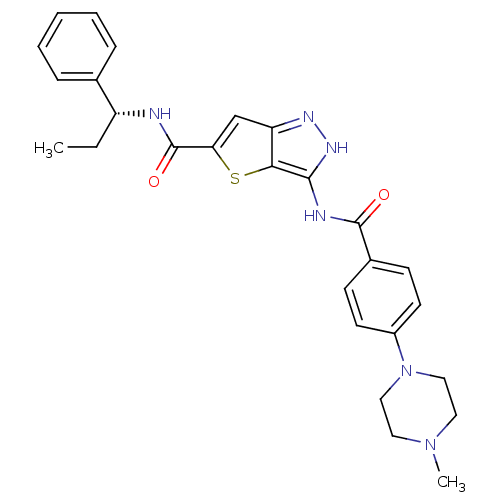
(3-({[4-(4-METHYLPIPERAZIN-1-YL)PHENYL]CARBONYL}AMI...)Show SMILES CC[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327923
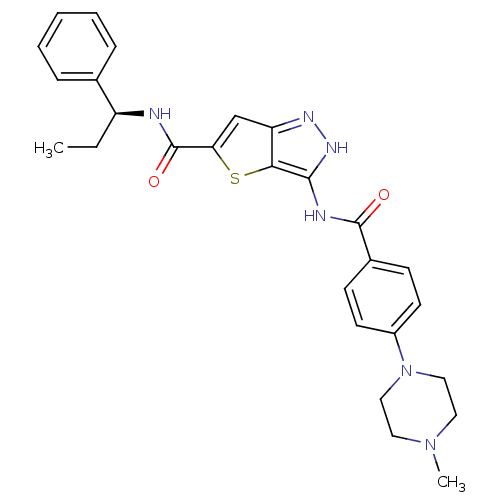
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327927
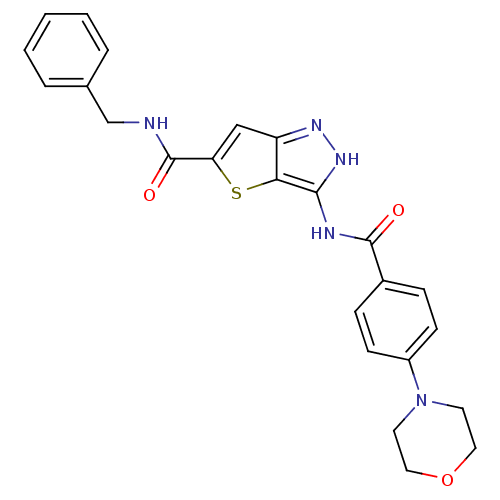
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(NCc1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 Show InChI InChI=1S/C24H23N5O3S/c30-23(17-6-8-18(9-7-17)29-10-12-32-13-11-29)26-22-21-19(27-28-22)14-20(33-21)24(31)25-15-16-4-2-1-3-5-16/h1-9,14H,10-13,15H2,(H,25,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327915
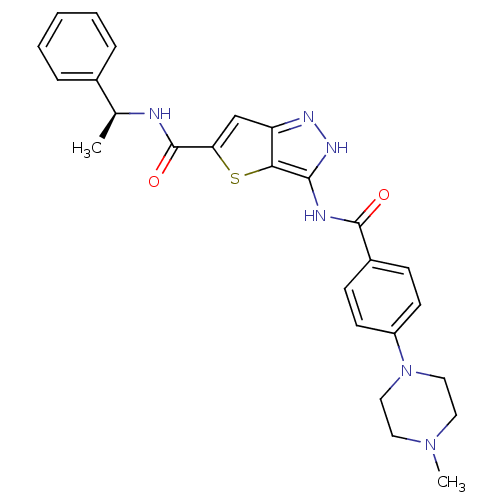
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12985
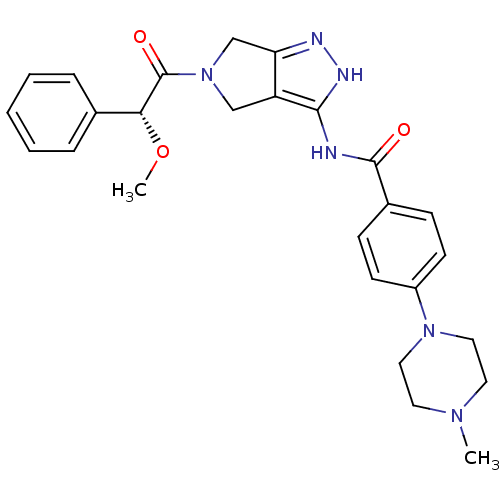
(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579781

(CHEMBL5094847)Show SMILES CC1(C)Cc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2C(C)(C)N1 |r,wU:13.16,10.9,(21.59,-25.94,;22.93,-25.18,;21.6,-24.4,;23.25,-26.69,;24.71,-27.16,;25.32,-28.56,;26.86,-28.42,;27.89,-29.57,;29.4,-29.25,;30.42,-30.4,;29.88,-27.79,;28.86,-26.64,;29.34,-25.18,;30.84,-24.87,;31.88,-26.01,;31.39,-27.48,;31.32,-23.41,;30.42,-22.16,;31.32,-20.92,;32.79,-21.4,;34.12,-20.62,;34.11,-19.08,;35.45,-21.39,;35.46,-22.94,;36.79,-23.7,;34.12,-23.71,;32.78,-22.94,;27.19,-26.9,;25.85,-26.13,;25.53,-24.64,;25.93,-23.14,;27.02,-24.23,;24.08,-24.16,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50327912
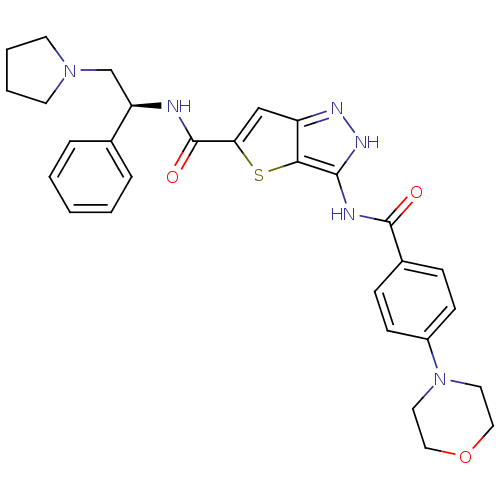
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579779
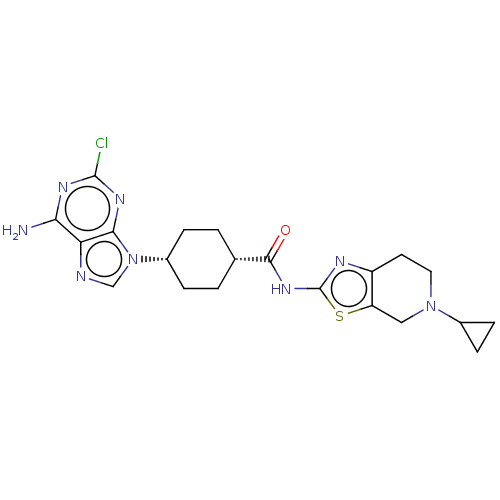
(CHEMBL5091519)Show SMILES Cl.Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2CCN(Cc2s1)C1CC1 |r,wU:12.12,15.19,(65.78,-4.32,;78.94,-3.56,;78.95,-5.1,;80.28,-5.86,;80.29,-7.41,;81.63,-8.18,;78.95,-8.19,;77.62,-7.41,;76.15,-7.89,;75.25,-6.64,;76.15,-5.4,;77.62,-5.87,;75.67,-9.35,;74.17,-9.66,;73.69,-11.12,;74.71,-12.27,;76.22,-11.96,;76.71,-10.49,;74.23,-13.74,;75.25,-14.89,;72.72,-14.05,;71.69,-12.9,;70.15,-13.04,;69.54,-11.64,;68.08,-11.17,;67.76,-9.66,;68.91,-8.64,;70.37,-9.12,;70.68,-10.61,;72.01,-11.38,;68.59,-7.13,;69.08,-5.67,;67.57,-5.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329928

(1-(2-methoxyethyl)-8-(5-(piperazin-1-yl)-2-(triflu...)Show SMILES COCCn1nc(C(N)=O)c2CCc3cnc(Nc4cc(ccc4OC(F)(F)F)N4CCN(C)CC4)nc3-c12 Show InChI InChI=1S/C25H29F3N8O3/c1-34-7-9-35(10-8-34)16-4-6-19(39-25(26,27)28)18(13-16)31-24-30-14-15-3-5-17-21(23(29)37)33-36(11-12-38-2)22(17)20(15)32-24/h4,6,13-14H,3,5,7-12H2,1-2H3,(H2,29,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327926
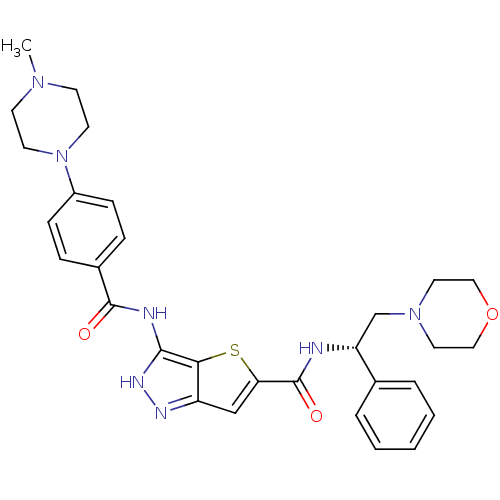
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)N[C@H](CN1CCOCC1)c1ccccc1 |r| Show InChI InChI=1S/C30H35N7O3S/c1-35-11-13-37(14-12-35)23-9-7-22(8-10-23)29(38)32-28-27-24(33-34-28)19-26(41-27)30(39)31-25(21-5-3-2-4-6-21)20-36-15-17-40-18-16-36/h2-10,19,25H,11-18,20H2,1H3,(H,31,39)(H2,32,33,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579773
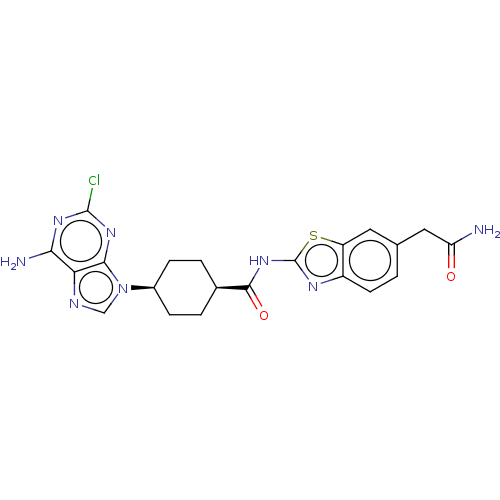
(CHEMBL5079663)Show SMILES NC(=O)Cc1ccc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2c1 |r,wU:16.19,13.12,(25.2,-43.33,;25.51,-44.84,;26.98,-45.33,;24.36,-45.87,;24.68,-47.38,;23.53,-48.41,;23.85,-49.9,;25.3,-50.38,;25.92,-51.79,;27.46,-51.64,;28.48,-52.79,;29.99,-52.48,;31.01,-53.63,;30.47,-51.02,;29.45,-49.87,;29.93,-48.41,;31.44,-48.1,;32.47,-49.24,;31.98,-50.7,;31.91,-46.64,;31.01,-45.39,;31.92,-44.14,;33.38,-44.62,;34.71,-43.85,;34.7,-42.3,;36.05,-44.61,;36.05,-46.16,;37.39,-46.93,;34.72,-46.93,;33.38,-46.16,;27.78,-50.13,;26.44,-49.36,;26.13,-47.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579782
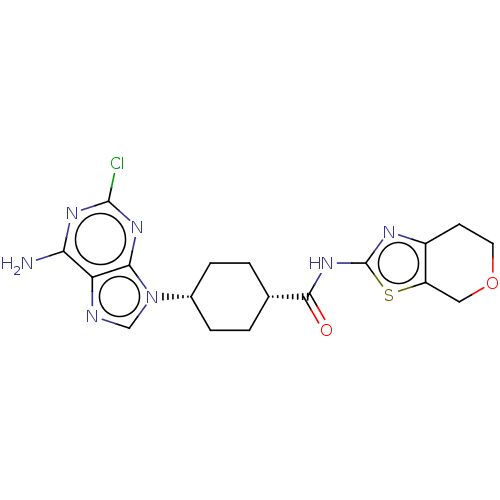
(CHEMBL5078600)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2CCOCc2s1 |r,wU:11.12,14.19,(53.68,-20.14,;53.69,-21.69,;55.03,-22.45,;55.03,-24,;56.37,-24.77,;53.69,-24.77,;52.36,-24,;50.89,-24.48,;49.99,-23.23,;50.9,-21.98,;52.36,-22.46,;50.41,-25.94,;48.91,-26.25,;48.43,-27.71,;49.45,-28.86,;50.96,-28.54,;51.45,-27.08,;48.97,-30.32,;49.99,-31.47,;47.46,-30.64,;46.43,-29.48,;44.89,-29.63,;44.28,-28.23,;42.82,-27.76,;42.51,-26.25,;43.65,-25.22,;45.11,-25.71,;45.42,-27.2,;46.76,-27.97,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579777
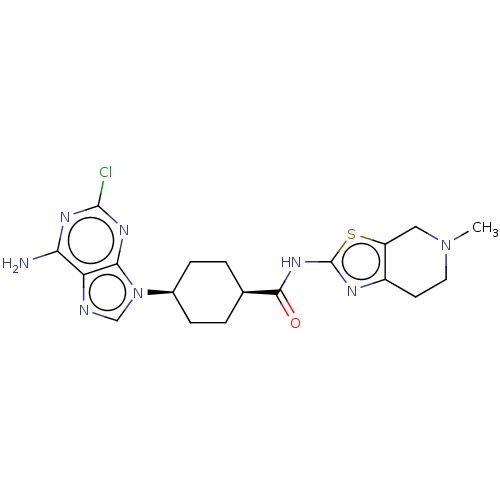
(CHEMBL5090932)Show SMILES CN1CCc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2C1 |r,wU:13.16,10.9,(24.36,-7.31,;24.67,-8.82,;23.53,-9.84,;23.84,-11.35,;25.3,-11.82,;25.92,-13.22,;27.46,-13.08,;28.48,-14.23,;29.99,-13.91,;31.02,-15.06,;30.47,-12.45,;29.45,-11.3,;29.93,-9.84,;31.44,-9.53,;32.47,-10.67,;31.98,-12.14,;31.92,-8.07,;31.01,-6.82,;31.92,-5.58,;33.38,-6.05,;34.71,-5.28,;34.71,-3.74,;36.05,-6.04,;36.05,-7.59,;37.39,-8.36,;34.72,-8.36,;33.38,-7.59,;27.78,-11.56,;26.44,-10.79,;26.13,-9.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579771
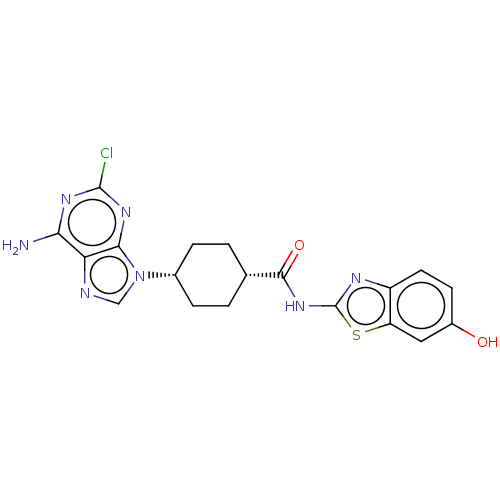
(CHEMBL5077194)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccc(O)cc2s1 |r,wU:11.12,14.19,(76.62,-21.9,;76.63,-23.44,;77.96,-24.2,;77.97,-25.75,;79.3,-26.52,;76.63,-26.53,;75.29,-25.76,;73.83,-26.23,;72.92,-24.98,;73.83,-23.74,;75.3,-24.22,;73.35,-27.69,;71.84,-28,;71.36,-29.46,;72.39,-30.61,;73.9,-30.3,;74.38,-28.83,;71.9,-32.08,;72.93,-33.23,;70.39,-32.39,;69.37,-31.24,;67.83,-31.38,;67.21,-29.97,;65.76,-29.49,;65.44,-28,;66.59,-26.97,;66.28,-25.46,;68.05,-27.45,;68.36,-28.95,;69.69,-29.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579769

(CHEMBL5075042)Show SMILES COc1ccc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2c1 |r,wU:14.17,11.10,(24.57,-24.01,;23.42,-25.03,;23.74,-26.54,;22.59,-27.57,;22.91,-29.07,;24.36,-29.54,;24.98,-30.95,;26.52,-30.81,;27.54,-31.96,;29.05,-31.65,;30.08,-32.8,;29.53,-30.18,;28.51,-29.03,;28.99,-27.57,;30.5,-27.27,;31.53,-28.4,;31.04,-29.87,;30.97,-25.8,;30.07,-24.55,;30.98,-23.31,;32.44,-23.79,;33.77,-23.01,;33.77,-21.47,;35.11,-23.77,;35.11,-25.33,;36.45,-26.09,;33.78,-26.1,;32.44,-25.33,;26.84,-29.3,;25.5,-28.53,;25.19,-27.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12984
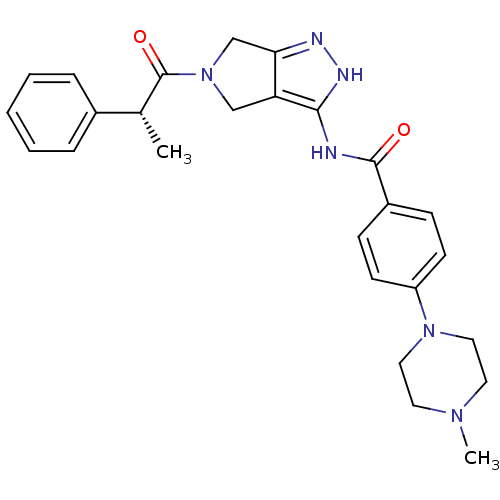
(4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...)Show SMILES C[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O2/c1-18(19-6-4-3-5-7-19)26(34)32-16-22-23(17-32)28-29-24(22)27-25(33)20-8-10-21(11-9-20)31-14-12-30(2)13-15-31/h3-11,18H,12-17H2,1-2H3,(H2,27,28,29,33)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329923
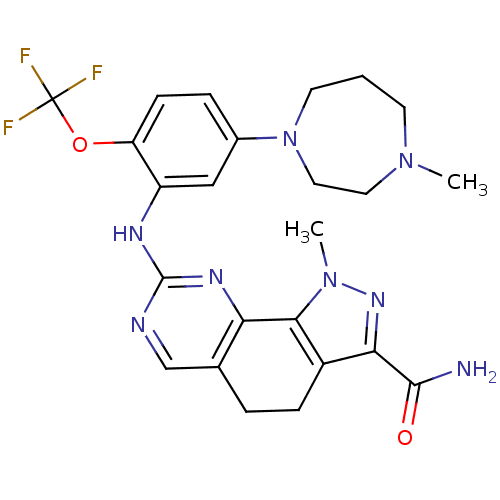
(1-methyl-8-(5-(4-methyl-1,4-diazepan-1-yl)-2-(trif...)Show SMILES CN1CCCN(CC1)c1ccc(OC(F)(F)F)c(Nc2ncc3CCc4c(nn(C)c4-c3n2)C(N)=O)c1 Show InChI InChI=1S/C24H27F3N8O2/c1-33-8-3-9-35(11-10-33)15-5-7-18(37-24(25,26)27)17(12-15)30-23-29-13-14-4-6-16-20(22(28)36)32-34(2)21(16)19(14)31-23/h5,7,12-13H,3-4,6,8-11H2,1-2H3,(H2,28,36)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327925
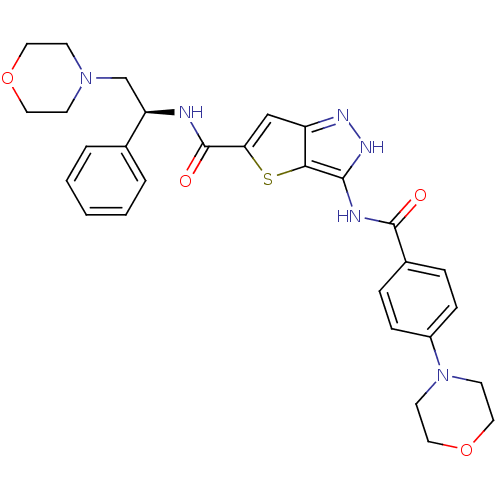
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCOCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O4S/c36-28(21-6-8-22(9-7-21)35-12-16-39-17-13-35)31-27-26-23(32-33-27)18-25(40-26)29(37)30-24(20-4-2-1-3-5-20)19-34-10-14-38-15-11-34/h1-9,18,24H,10-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327924
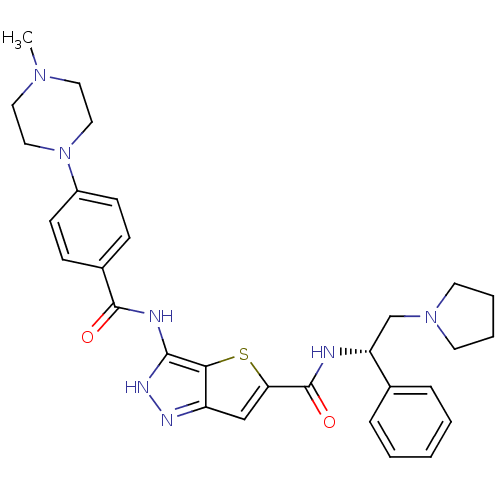
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)N[C@H](CN1CCCC1)c1ccccc1 |r| Show InChI InChI=1S/C30H35N7O2S/c1-35-15-17-37(18-16-35)23-11-9-22(10-12-23)29(38)32-28-27-24(33-34-28)19-26(40-27)30(39)31-25(20-36-13-5-6-14-36)21-7-3-2-4-8-21/h2-4,7-12,19,25H,5-6,13-18,20H2,1H3,(H,31,39)(H2,32,33,34,38)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12110
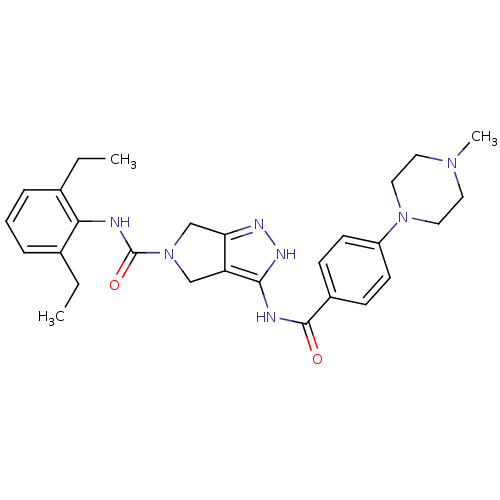
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 18 | 5-N-...)Show SMILES CCc1cccc(CC)c1NC(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1 Show InChI InChI=1S/C28H35N7O2/c1-4-19-7-6-8-20(5-2)25(19)29-28(37)35-17-23-24(18-35)31-32-26(23)30-27(36)21-9-11-22(12-10-21)34-15-13-33(3)14-16-34/h6-12H,4-5,13-18H2,1-3H3,(H,29,37)(H2,30,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12110

(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 18 | 5-N-...)Show SMILES CCc1cccc(CC)c1NC(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1 Show InChI InChI=1S/C28H35N7O2/c1-4-19-7-6-8-20(5-2)25(19)29-28(37)35-17-23-24(18-35)31-32-26(23)30-27(36)21-9-11-22(12-10-21)34-15-13-33(3)14-16-34/h6-12H,4-5,13-18H2,1-3H3,(H,29,37)(H2,30,31,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579780

(CHEMBL5089356)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2CCNCc2s1 |r,wU:11.12,14.19,(14.03,-18.46,;14.04,-20,;15.37,-20.77,;15.38,-22.32,;16.72,-23.09,;14.04,-23.09,;12.71,-22.32,;11.24,-22.79,;10.34,-21.55,;11.24,-20.3,;12.71,-20.78,;10.76,-24.26,;9.26,-24.57,;8.78,-26.03,;9.8,-27.18,;11.31,-26.86,;11.8,-25.4,;9.32,-28.64,;10.34,-29.79,;7.81,-28.95,;6.78,-27.8,;5.24,-27.95,;4.63,-26.54,;3.17,-26.08,;2.85,-24.57,;4,-23.54,;5.46,-24.02,;5.77,-25.52,;7.1,-26.29,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
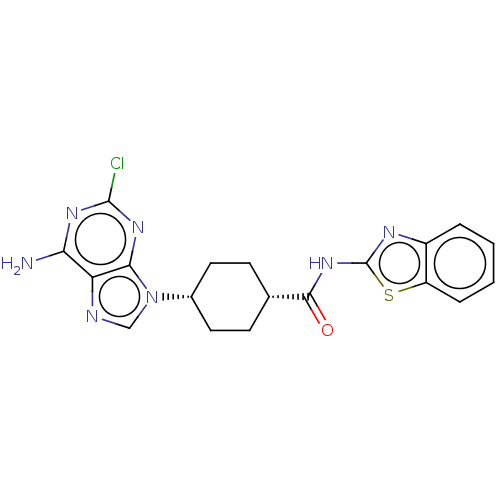
(Homo sapiens (Human)) | BDBM50579757
(CHEMBL5084844)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccccc2s1 |r,wU:11.12,14.19,(17.43,-18.47,;17.43,-20.01,;18.77,-20.78,;18.77,-22.33,;20.11,-23.1,;17.44,-23.1,;16.1,-22.33,;14.64,-22.8,;13.73,-21.56,;14.64,-20.31,;16.1,-20.79,;14.16,-24.27,;12.65,-24.58,;12.17,-26.03,;13.19,-27.19,;14.71,-26.87,;15.19,-25.4,;12.71,-28.65,;13.74,-29.8,;11.2,-28.96,;10.18,-27.81,;8.64,-27.96,;8.02,-26.55,;6.57,-26.07,;6.25,-24.57,;7.4,-23.55,;8.86,-24.03,;9.16,-25.53,;10.5,-26.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
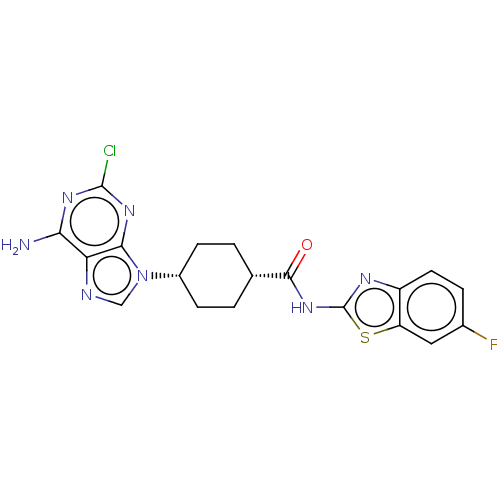
(Homo sapiens (Human)) | BDBM50579775
(CHEMBL5085262)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2ccc(F)cc2s1 |r,wU:11.12,14.19,(75.4,-43.26,;75.4,-44.81,;76.74,-45.57,;76.75,-47.12,;78.08,-47.89,;75.41,-47.89,;74.07,-47.12,;72.61,-47.6,;71.7,-46.35,;72.61,-45.1,;74.07,-45.58,;72.13,-49.06,;70.62,-49.37,;70.14,-50.83,;71.16,-51.98,;72.68,-51.67,;73.16,-50.2,;70.68,-53.44,;71.71,-54.59,;69.17,-53.76,;68.15,-52.61,;66.61,-52.75,;65.99,-51.34,;64.54,-50.86,;64.22,-49.37,;65.37,-48.34,;65.06,-46.83,;66.83,-48.82,;67.13,-50.32,;68.47,-51.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
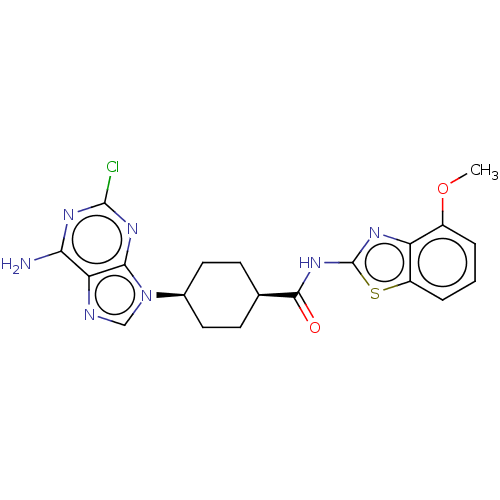
(Homo sapiens (Human)) | BDBM50579770
(CHEMBL5090132)Show SMILES COc1cccc2sc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)nc12 |r,wU:15.18,12.11,(43.36,-30.19,;44.82,-30.67,;45.97,-29.64,;45.66,-28.15,;46.8,-27.12,;48.26,-27.6,;48.57,-29.1,;49.9,-29.87,;49.58,-31.39,;50.61,-32.54,;52.12,-32.22,;53.14,-33.37,;52.6,-30.76,;51.58,-29.61,;52.05,-28.15,;53.56,-27.84,;54.6,-28.98,;54.11,-30.45,;54.04,-26.38,;53.14,-25.13,;54.04,-23.88,;55.51,-24.36,;56.84,-23.59,;56.83,-22.04,;58.17,-24.35,;58.18,-25.9,;59.52,-26.67,;56.84,-26.67,;55.51,-25.9,;48.04,-31.53,;47.42,-30.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579774

(CHEMBL5081222)Show SMILES NC(=O)c1ccc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(Cl)nc34)sc2c1 |r,wU:15.18,12.11,(43.29,-45.93,;44.75,-46.41,;45.9,-45.38,;45.07,-47.92,;43.92,-48.94,;44.23,-50.44,;45.68,-50.92,;46.3,-52.33,;47.84,-52.18,;48.87,-53.33,;50.38,-53.02,;51.4,-54.17,;50.86,-51.56,;49.84,-50.4,;50.32,-48.95,;51.82,-48.64,;52.86,-49.77,;52.37,-51.24,;52.3,-47.17,;51.4,-45.93,;52.31,-44.68,;53.77,-45.16,;55.1,-44.38,;55.09,-42.84,;56.44,-45.15,;56.44,-46.7,;57.78,-47.47,;55.1,-47.47,;53.77,-46.7,;48.16,-50.67,;46.83,-49.9,;46.52,-48.4,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
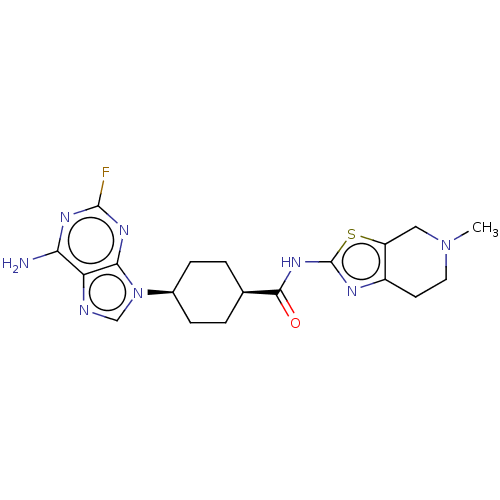
(Homo sapiens (Human)) | BDBM50579785
(CHEMBL5075110)Show SMILES CN1CCc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(F)nc34)sc2C1 |r,wU:13.16,10.9,(23.79,-42.21,;24.11,-43.72,;22.96,-44.75,;23.28,-46.25,;24.73,-46.72,;25.35,-48.13,;26.89,-47.98,;27.91,-49.13,;29.42,-48.82,;30.45,-49.97,;29.91,-47.36,;28.88,-46.2,;29.36,-44.75,;30.87,-44.44,;31.9,-45.57,;31.42,-47.04,;31.35,-42.97,;30.45,-41.73,;31.35,-40.48,;32.82,-40.96,;34.15,-40.18,;34.14,-38.64,;35.48,-40.95,;35.49,-42.5,;36.82,-43.27,;34.15,-43.27,;32.81,-42.5,;27.21,-46.47,;25.88,-45.7,;25.56,-44.2,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
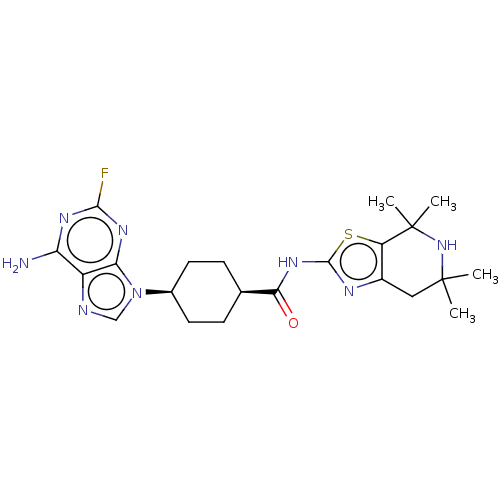
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579786
(CHEMBL5093152)Show SMILES CC1(C)Cc2nc(NC(=O)[C@H]3CC[C@H](CC3)n3cnc4c(N)nc(F)nc34)sc2C(C)(C)N1 |r,wU:13.16,10.9,(42.91,-44.68,;44.24,-43.92,;42.91,-43.14,;44.56,-45.43,;46.02,-45.89,;46.64,-47.3,;48.17,-47.15,;49.2,-48.3,;50.71,-47.99,;51.73,-49.14,;51.19,-46.53,;50.17,-45.38,;50.65,-43.92,;52.15,-43.61,;53.19,-44.75,;52.7,-46.21,;52.63,-42.15,;51.73,-40.9,;52.64,-39.66,;54.1,-40.13,;55.43,-39.36,;55.42,-37.82,;56.76,-40.12,;56.77,-41.67,;58.1,-42.44,;55.43,-42.44,;54.1,-41.67,;48.5,-45.64,;47.16,-44.87,;46.84,-43.37,;47.24,-41.88,;48.33,-42.97,;45.39,-42.89,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327923

(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
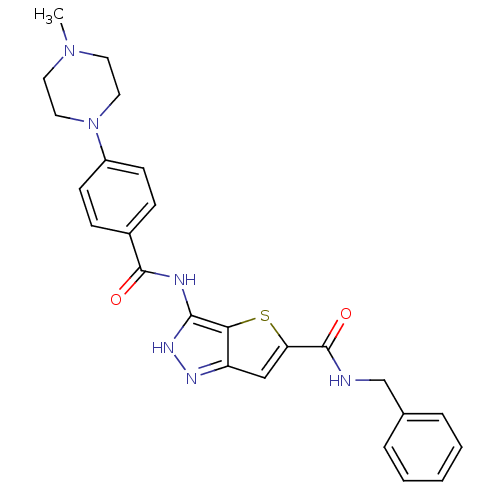
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327922
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2cc(sc12)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H26N6O2S/c1-30-11-13-31(14-12-30)19-9-7-18(8-10-19)24(32)27-23-22-20(28-29-23)15-21(34-22)25(33)26-16-17-5-3-2-4-6-17/h2-10,15H,11-14,16H2,1H3,(H,26,33)(H2,27,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
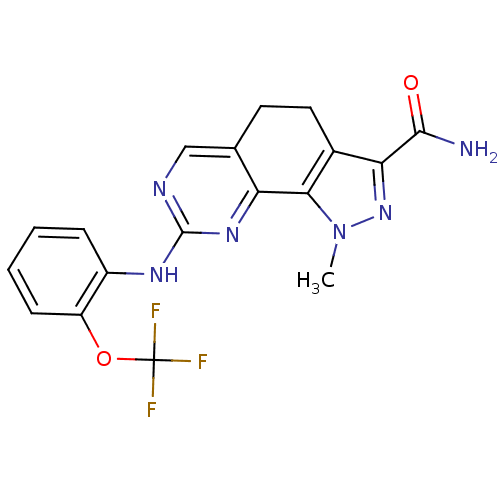
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50329932
(1-methyl-8-(2-(trifluoromethoxy)phenylamino)-4,5-d...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4ccccc4OC(F)(F)F)nc3-c12 Show InChI InChI=1S/C18H15F3N6O2/c1-27-15-10(14(26-27)16(22)28)7-6-9-8-23-17(25-13(9)15)24-11-4-2-3-5-12(11)29-18(19,20)21/h2-5,8H,6-7H2,1H3,(H2,22,28)(H,23,24,25) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK3 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
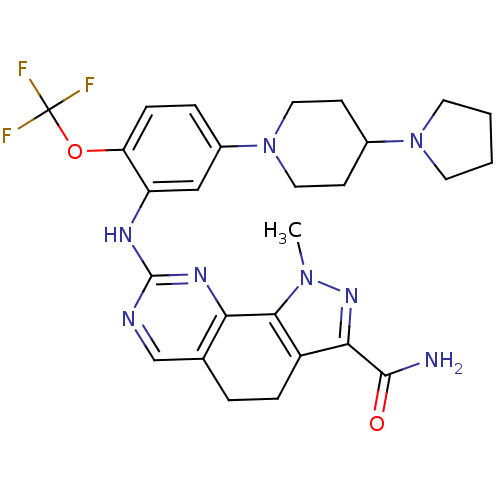
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50329924
(1-methyl-8-(5-(4-(pyrrolidin-1-yl)piperidin-1-yl)-...)Show SMILES Cn1nc(C(N)=O)c2CCc3cnc(Nc4cc(ccc4OC(F)(F)F)N4CCC(CC4)N4CCCC4)nc3-c12 Show InChI InChI=1S/C27H31F3N8O2/c1-36-24-19(23(35-36)25(31)39)6-4-16-15-32-26(34-22(16)24)33-20-14-18(5-7-21(20)40-27(28,29)30)38-12-8-17(9-13-38)37-10-2-3-11-37/h5,7,14-15,17H,2-4,6,8-13H2,1H3,(H2,31,39)(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 |
Bioorg Med Chem Lett 20: 6489-94 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.060
BindingDB Entry DOI: 10.7270/Q2VT1T39 |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579772
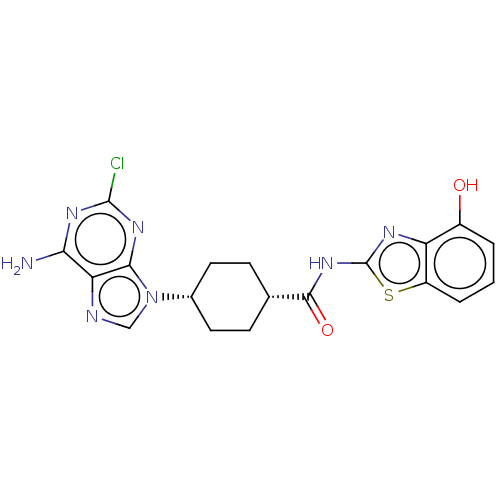
(CHEMBL5088776)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1CC[C@@H](CC1)C(=O)Nc1nc2c(O)cccc2s1 |r,wU:11.12,14.19,(14.05,-42.03,;14.05,-43.58,;15.39,-44.34,;15.4,-45.89,;16.73,-46.66,;14.06,-46.66,;12.72,-45.89,;11.26,-46.37,;10.35,-45.12,;11.26,-43.87,;12.72,-44.35,;10.78,-47.83,;9.27,-48.14,;8.79,-49.6,;9.82,-50.75,;11.33,-50.44,;11.81,-48.97,;9.33,-52.21,;10.36,-53.36,;7.82,-52.53,;6.8,-51.38,;5.26,-51.52,;4.64,-50.11,;3.19,-49.63,;2.04,-50.66,;2.87,-48.14,;4.02,-47.11,;5.48,-47.59,;5.79,-49.09,;7.12,-49.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Choline kinase alpha
(Homo sapiens (Human)) | BDBM50579784
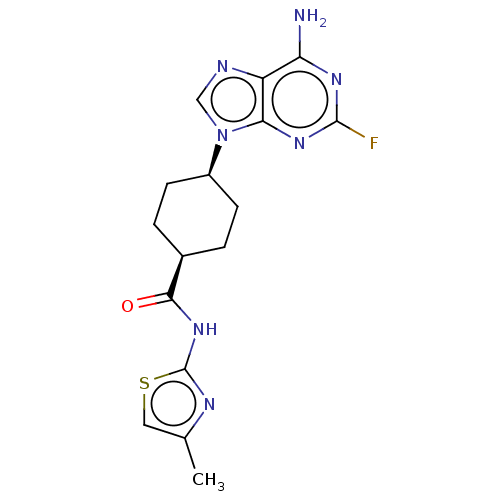
(CHEMBL5091201)Show SMILES Cc1csc(NC(=O)[C@H]2CC[C@H](CC2)n2cnc3c(N)nc(F)nc23)n1 |r,wU:11.14,8.7,(4.23,-45.63,;5.68,-46.1,;6.83,-45.08,;8.16,-45.85,;7.84,-47.36,;8.87,-48.51,;10.37,-48.2,;11.4,-49.35,;10.86,-46.74,;9.83,-45.58,;10.31,-44.13,;11.82,-43.82,;12.86,-44.95,;12.37,-46.42,;12.3,-42.35,;11.4,-41.11,;12.3,-39.86,;13.77,-40.34,;15.1,-39.56,;15.09,-38.02,;16.43,-40.33,;16.44,-41.88,;17.77,-42.65,;15.1,-42.65,;13.76,-41.88,;6.3,-47.51,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ChoKalpha (unknown origin) by HTS assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128310
BindingDB Entry DOI: 10.7270/Q27H1PFF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of KIT |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data