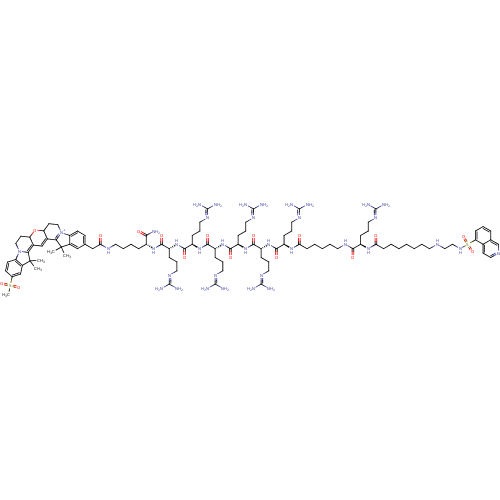| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Ribosomal protein S6 kinase alpha-5 |
|---|
| Ligand | BDBM50382224 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_816449 (CHEMBL2024936) |
|---|
| Kd | 12±n/a nM |
|---|
| Citation |  Lavogina, D; Kalind, K; Bredihhina, J; Hurt, M; Vaasa, A; Kasari, M; Enkvist, E; Raidaru, G; Uri, A Conjugates of 5-isoquinolinesulfonylamides and oligo-D-arginine possess high affinity and selectivity towards Rho kinase (ROCK). Bioorg Med Chem Lett22:3425-30 (2012) [PubMed] Article Lavogina, D; Kalind, K; Bredihhina, J; Hurt, M; Vaasa, A; Kasari, M; Enkvist, E; Raidaru, G; Uri, A Conjugates of 5-isoquinolinesulfonylamides and oligo-D-arginine possess high affinity and selectivity towards Rho kinase (ROCK). Bioorg Med Chem Lett22:3425-30 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Ribosomal protein S6 kinase alpha-5 |
|---|
| Name: | Ribosomal protein S6 kinase alpha-5 |
|---|
| Synonyms: | 90 kDa ribosomal protein S6 kinase 5 | KS6A5_HUMAN | MSK1 | Mitogen- and Stress-Activated Protein Kinase 1 (MSK1) | Nuclear mitogen- and stress-activated protein kinase 1 | Nuclear mitogen- and stress-activated protein kinase 1 (MSK1) | RPS6KA5 | RPS6KA5(Kin.Dom.2 - C-terminal) | RSK-like protein kinase | RSKL | Ribosomal protein S6 kinase | Ribosomal protein S6 kinase alpha 5 | Ribosomal protein S6 kinase alpha-5 |
|---|
| Type: | Serine/threonine-protein kinase |
|---|
| Mol. Mass.: | 89874.44 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 802 |
|---|
| Sequence: | MEEEGGSSGGAAGTSADGGDGGEQLLTVKHELRTANLTGHAEKVGIENFELLKVLGTGAY
GKVFLVRKISGHDTGKLYAMKVLKKATIVQKAKTTEHTRTERQVLEHIRQSPFLVTLHYA
FQTETKLHLILDYINGGELFTHLSQRERFTEHEVQIYVGEIVLALEHLHKLGIIYRDIKL
ENILLDSNGHVVLTDFGLSKEFVADETERAYSFCGTIEYMAPDIVRGGDSGHDKAVDWWS
LGVLMYELLTGASPFTVDGEKNSQAEISRRILKSEPPYPQEMSALAKDLIQRLLMKDPKK
RLGCGPRDADEIKEHLFFQKINWDDLAAKKVPAPFKPVIRDELDVSNFAEEFTEMDPTYS
PAALPQSSEKLFQGYSFVAPSILFKRNAAVIDPLQFHMGVERPGVTNVARSAMMKDSPFY
QHYDLDLKDKPLGEGSFSICRKCVHKKSNQAFAVKIISKRMEANTQKEITALKLCEGHPN
IVKLHEVFHDQLHTFLVMELLNGGELFERIKKKKHFSETEASYIMRKLVSAVSHMHDVGV
VHRDLKPENLLFTDENDNLEIKIIDFGFARLKPPDNQPLKTPCFTLHYAAPELLNQNGYD
ESCDLWSLGVILYTMLSGQVPFQSHDRSLTCTSAVEIMKKIKKGDFSFEGEAWKNVSQEA
KDLIQGLLTVDPNKRLKMSGLRYNEWLQDGSQLSSNPLMTPDILGSSGAAVHTCVKATFH
AFNKYKREGFCLQNVDKAPLAKRRKMKKTSTSTETRSSSSESSHSSSSHSHGKTTPTKTL
QPSNPADSNNPETLFQFSDSVA
|
|
|
|---|
| BDBM50382224 |
|---|
| n/a |
|---|
| Name | BDBM50382224 |
|---|
| Synonyms: | CHEMBL2023841 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C105H168N37O16S2 |
|---|
| Mol. Mass. | 2268.825 |
|---|
| SMILES | [#6]C1([#6])[#6]-2=[#6]-3-[#6]=[#6]-4-[#6](-[#6]-[#6]-[#7+]-5=[#6]-4C([#6])([#6])c4cc(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-[#7]S(=O)(=O)c6cccc7cnccc67)-[#6](-[#7])=O)ccc-54)-[#8]-[#6]-3-[#6]-[#6]-[#7]-2-c2ccc(cc12)S([#6])(=O)=O |r,c:3,5,10| |
|---|
| Structure | 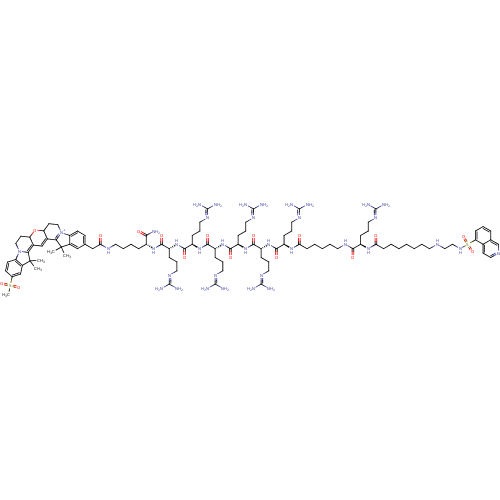
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lavogina, D; Kalind, K; Bredihhina, J; Hurt, M; Vaasa, A; Kasari, M; Enkvist, E; Raidaru, G; Uri, A Conjugates of 5-isoquinolinesulfonylamides and oligo-D-arginine possess high affinity and selectivity towards Rho kinase (ROCK). Bioorg Med Chem Lett22:3425-30 (2012) [PubMed] Article
Lavogina, D; Kalind, K; Bredihhina, J; Hurt, M; Vaasa, A; Kasari, M; Enkvist, E; Raidaru, G; Uri, A Conjugates of 5-isoquinolinesulfonylamides and oligo-D-arginine possess high affinity and selectivity towards Rho kinase (ROCK). Bioorg Med Chem Lett22:3425-30 (2012) [PubMed] Article