 Found 9157 hits with Last Name = 'uri' and Initial = 'a'
Found 9157 hits with Last Name = 'uri' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599153
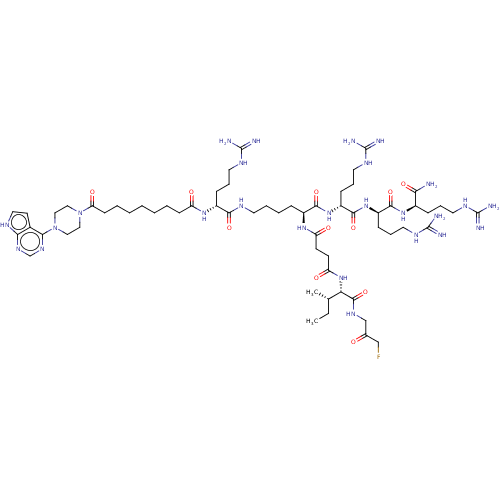
(CHEMBL5196521)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM109209
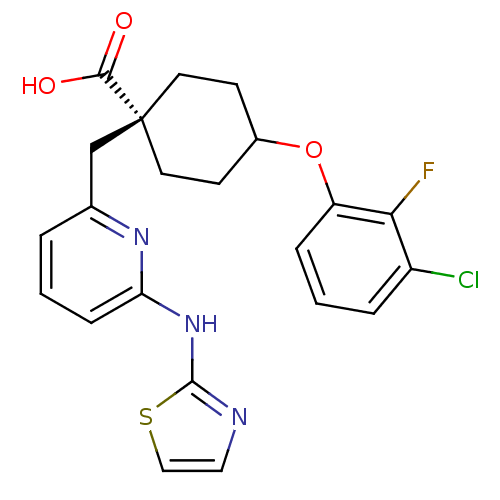
(VX-689)Show SMILES OC(=O)[C@]1(Cc2cccc(Nc3nccs3)n2)CCC(CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:3.3,(2.79,-10.07,;2.79,-8.53,;4.12,-7.76,;1.46,-7.76,;1.46,-9.3,;.12,-10.07,;.12,-11.61,;-1.21,-12.38,;-2.54,-11.61,;-2.54,-10.07,;-3.88,-9.3,;-3.88,-7.76,;-5.12,-6.85,;-4.65,-5.39,;-3.11,-5.39,;-2.63,-6.85,;-1.21,-9.3,;2.79,-6.99,;2.79,-5.45,;1.46,-4.68,;.12,-5.45,;.12,-6.99,;1.46,-3.14,;.12,-2.37,;-1.21,-3.14,;-2.54,-2.37,;-2.54,-.83,;-1.21,-.05,;-1.21,1.49,;.12,-.83,;1.46,-.06,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15?,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0155 | -62.7 | 3.88 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599152
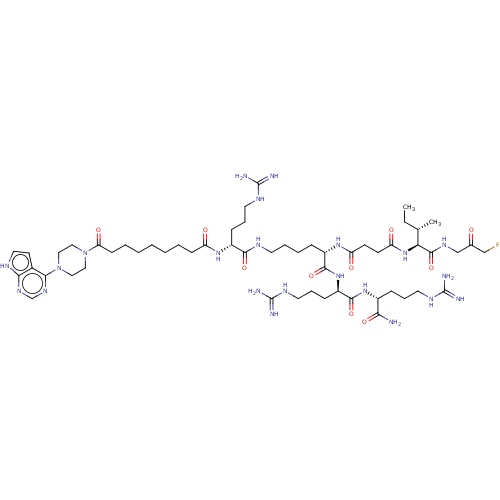
(CHEMBL5179794)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis |
J Med Chem 58: 6237-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00795
BindingDB Entry DOI: 10.7270/Q2H133S9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50053929
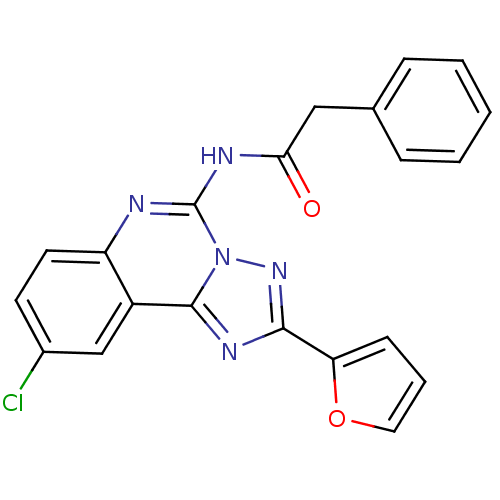
(CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...)Show SMILES Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 Show InChI InChI=1S/C21H14ClN5O2/c22-14-8-9-16-15(12-14)20-25-19(17-7-4-10-29-17)26-27(20)21(23-16)24-18(28)11-13-5-2-1-3-6-13/h1-10,12H,11H2,(H,23,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591169
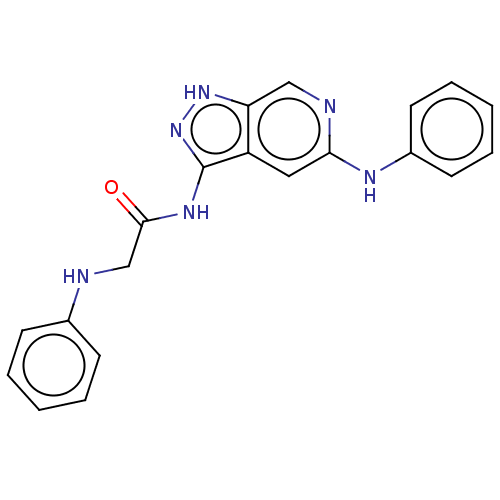
(CHEMBL5201810)Show SMILES O=C(CNc1ccccc1)Nc1n[nH]c2cnc(Nc3ccccc3)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.136 | -57.3 | 34.2 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50104639

(CHEMBL3593364)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](=[#6]\[#6]=[#6]-3/[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-3)-[#6@@H](-[#6])-[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6]C([#6])([#6])[#8] |r| Show InChI InChI=1S/C27H46O3/c1-18-12-14-27(5)24(19(2)7-6-13-26(3,4)30)10-11-25(27)23(18)9-8-20-15-21(28)17-22(29)16-20/h8-9,18-19,21-22,24-25,28-30H,6-7,10-17H2,1-5H3/b23-9+/t18-,19+,21+,22+,24+,25-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis |
J Med Chem 58: 6237-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00795
BindingDB Entry DOI: 10.7270/Q2H133S9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599149
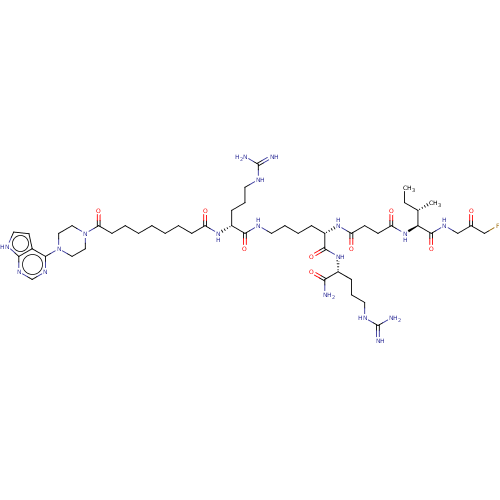
(CHEMBL5174535)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50107863
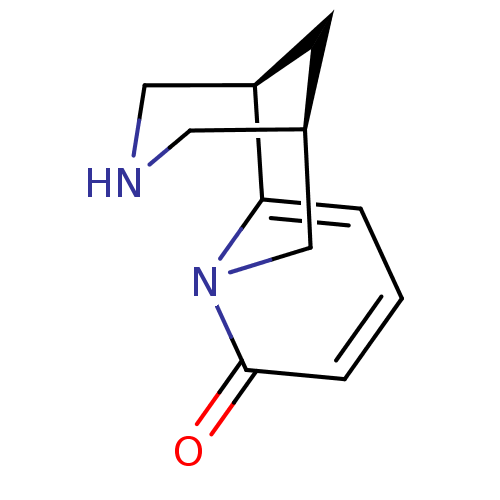
((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
Aurora kinase A/Targeting protein for Xklp2 [1-43]
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.86 | -50.7 | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Aurora kinase A/Targeting protein for Xklp2 [1-43]
(Homo sapiens (Human)) | BDBM109209

(VX-689)Show SMILES OC(=O)[C@]1(Cc2cccc(Nc3nccs3)n2)CCC(CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:3.3,(2.79,-10.07,;2.79,-8.53,;4.12,-7.76,;1.46,-7.76,;1.46,-9.3,;.12,-10.07,;.12,-11.61,;-1.21,-12.38,;-2.54,-11.61,;-2.54,-10.07,;-3.88,-9.3,;-3.88,-7.76,;-5.12,-6.85,;-4.65,-5.39,;-3.11,-5.39,;-2.63,-6.85,;-1.21,-9.3,;2.79,-6.99,;2.79,-5.45,;1.46,-4.68,;.12,-5.45,;.12,-6.99,;1.46,-3.14,;.12,-2.37,;-1.21,-3.14,;-2.54,-2.37,;-2.54,-.83,;-1.21,-.05,;-1.21,1.49,;.12,-.83,;1.46,-.06,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15?,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.17 | -50.3 | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
University of Tartu, Ravila 14A, 50411 Tartu (Estonia)
| Assay Description
Threefold dilution series of inhibitors in assay buffer were prepared in wells of a microtiter plate (final concentration stating from 2uM), and TAMR... |
Chembiochem 15: 443-50 (2014)
Article DOI: 10.1002/cbic.201300613
BindingDB Entry DOI: 10.7270/Q2028Q58 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Dihydrofolate reductase enzyme purified from Plasmodium berghei. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot |
Eur J Med Chem 97: 181-9 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.055
BindingDB Entry DOI: 10.7270/Q2P84DM4 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50599151
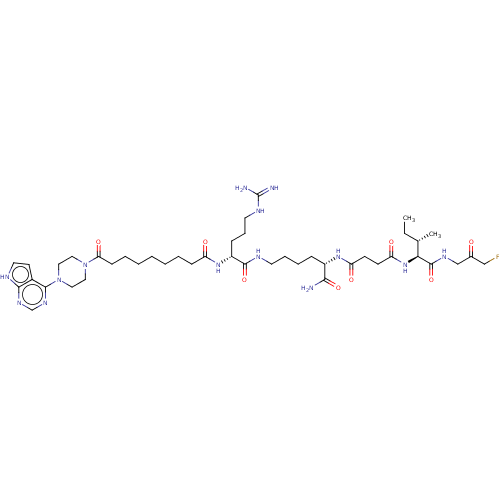
(CHEMBL5172486)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)N[C@@H](CCCCNC(=O)[C@@H](CCCNC(N)=N)NC(=O)CCCCCCCC(=O)N1CCN(CC1)c1ncnc2[nH]ccc12)C(N)=O)C(=O)NCC(=O)CF |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00067
BindingDB Entry DOI: 10.7270/Q2NK3K3W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50591158
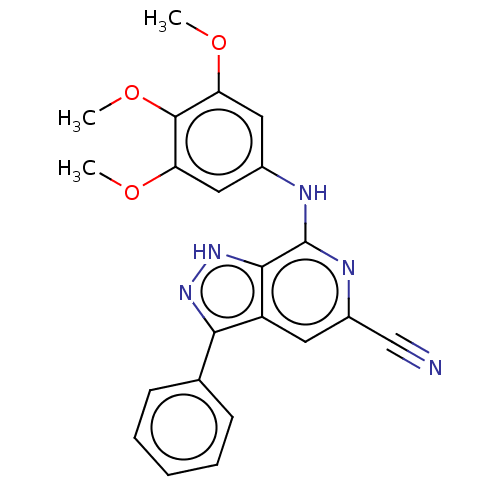
(CHEMBL5181728)Show SMILES COc1cc(Nc2nc(cc3c(n[nH]c23)-c2ccccc2)C#N)cc(OC)c1OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
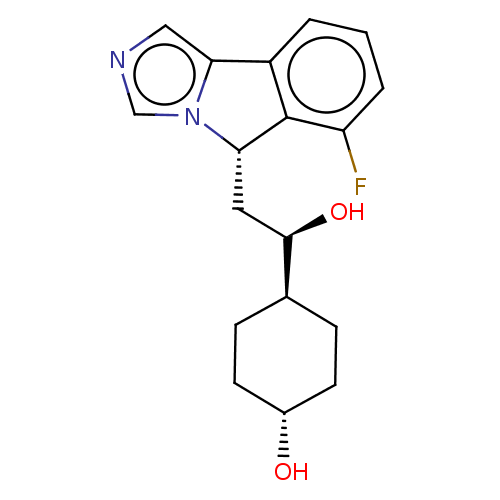
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation
Curated by ChEMBL
| Assay Description
Inhibition of purified human IDO1 using varying levels of L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measur... |
J Med Chem 62: 6705-6733 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00662
BindingDB Entry DOI: 10.7270/Q21G0QNZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nicotinic acetylcholine receptor
(RAT) | BDBM50023330
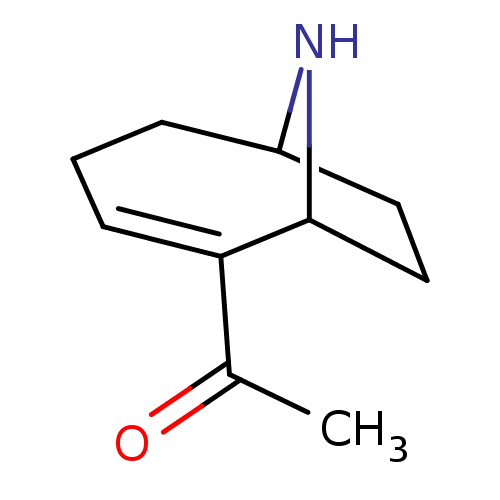
(1-(9-Aza-bicyclo[4.2.1]non-2-en-2-yl)-ethanone | 1...)Show InChI InChI=1S/C10H15NO/c1-7(12)9-4-2-3-8-5-6-10(9)11-8/h4,8,10-11H,2-3,5-6H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592974
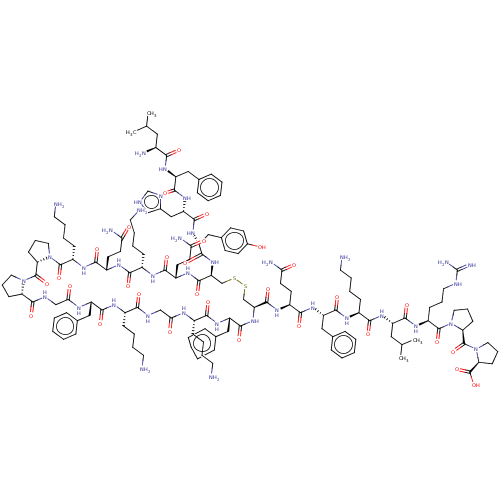
(CHEMBL5180727)Show SMILES [H][C@@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC2=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592982
(CHEMBL5176092)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592982

(CHEMBL5176092)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50072996
(3-[5-((E)-2-Diethylcarbamoyl-1-methyl-vinyl)-2-(1-...)Show SMILES CCN(CC)C(=O)\C=C(/C)c1ccc(OC(C)c2ccccc2)c(CCC(O)=O)c1 Show InChI InChI=1S/C25H31NO4/c1-5-26(6-2)24(27)16-18(3)21-12-14-23(22(17-21)13-15-25(28)29)30-19(4)20-10-8-7-9-11-20/h7-12,14,16-17,19H,5-6,13,15H2,1-4H3,(H,28,29)/b18-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Leukotriene B4 receptor antagonistic activity was measured by the inhibition of LTB4 induced [Ca2+] release from human PMNs |
J Med Chem 42: 164-72 (1999)
Article DOI: 10.1021/jm980540v
BindingDB Entry DOI: 10.7270/Q26972QG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591158

(CHEMBL5181728)Show SMILES COc1cc(Nc2nc(cc3c(n[nH]c23)-c2ccccc2)C#N)cc(OC)c1OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267028

(CHEMBL4073525)Show SMILES Cc1cc(OCc2ccccc2)ccc1Oc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C27H26F3NO4/c1-18-13-24(34-17-19-5-3-2-4-6-19)11-12-25(18)35-23-9-7-21(8-10-23)31-16-20(27(28,29)30)14-22(31)15-26(32)33/h2-13,20,22H,14-17H2,1H3,(H,32,33)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191
(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50591160
(CHEMBL5173159)Show SMILES COc1cc(Nc2nc(cc3c(n[nH]c23)C(C)C)C#N)cc(OC)c1OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592991

(CHEMBL5182977)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592991

(CHEMBL5182977)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 1
(Homo sapiens (Human)) | BDBM50267031

(CHEMBL4079930)Show SMILES Cc1c(Cl)cccc1Oc1ccc(cc1)N1C[C@H](C[C@H]1CC(O)=O)C(F)(F)F |r| Show InChI InChI=1S/C20H19ClF3NO3/c1-12-17(21)3-2-4-18(12)28-16-7-5-14(6-8-16)25-11-13(20(22,23)24)9-15(25)10-19(26)27/h2-8,13,15H,9-11H2,1H3,(H,26,27)/t13-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay |
J Med Chem 60: 1417-1431 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01559
BindingDB Entry DOI: 10.7270/Q25X2CD5 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50405457

(CHEMBL5283903)Show SMILES CC(C)Cn1c2ccc3[nH]c(nc3c2c(=O)n(C)c1=O)C(C)(C)C Show InChI InChI=1S/C18H24N4O2/c1-10(2)9-22-12-8-7-11-14(20-16(19-11)18(3,4)5)13(12)15(23)21(6)17(22)24/h7-8,10H,9H2,1-6H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human platelet Thromboxane synthetase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592978
(CHEMBL5190661)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592972
(CHEMBL5198158)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CSC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592978

(CHEMBL5190661)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50104640
(CHEMBL3593365)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(=C\C=C3\C[C@@H](O)C[C@H](O)C3=C)[C@H](C)CC[C@]12C)[C@H](C)CCCC(C)(C)O |r| Show InChI InChI=1S/C28H46O3/c1-18-13-15-28(6)24(19(2)8-7-14-27(4,5)31)11-12-25(28)23(18)10-9-21-16-22(29)17-26(30)20(21)3/h9-10,18-19,22,24-26,29-31H,3,7-8,11-17H2,1-2,4-6H3/b21-9-,23-10+/t18-,19-,22-,24-,25+,26+,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis |
J Med Chem 58: 6237-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00795
BindingDB Entry DOI: 10.7270/Q2H133S9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50104641
(CHEMBL3593366)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](=[#6]\[#6]=[#6]-3/[#6]-[#6@@H](-[#8])-[#6]-[#6@H](-[#8])-[#6]-3)-[#6](=[#6])-[#6]-[#6][C@]12[#6])[#6@H](-[#6])-[#6]-[#6]-[#6]C([#6])([#6])[#8] |r| Show InChI InChI=1S/C27H44O3/c1-18-12-14-27(5)24(19(2)7-6-13-26(3,4)30)10-11-25(27)23(18)9-8-20-15-21(28)17-22(29)16-20/h8-9,19,21-22,24-25,28-30H,1,6-7,10-17H2,2-5H3/b23-9+/t19-,21-,22-,24-,25+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis |
J Med Chem 58: 6237-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00795
BindingDB Entry DOI: 10.7270/Q2H133S9 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50591164
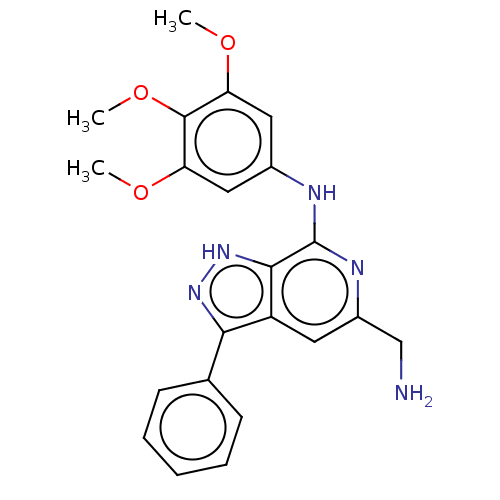
(CHEMBL5196781)Show SMILES COc1cc(Nc2nc(CN)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50592974

(CHEMBL5180727)Show SMILES [H][C@@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC2=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(O)=O)NC(=O)[C@@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CC(C)C |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50597191

(CHEMBL5195631) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50592992
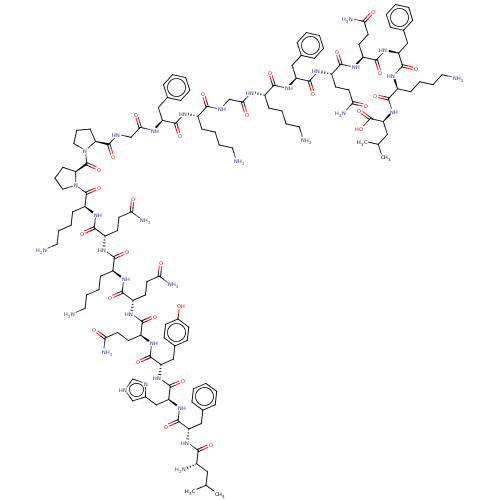
(CHEMBL5195766)Show SMILES CC(C)C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50592979
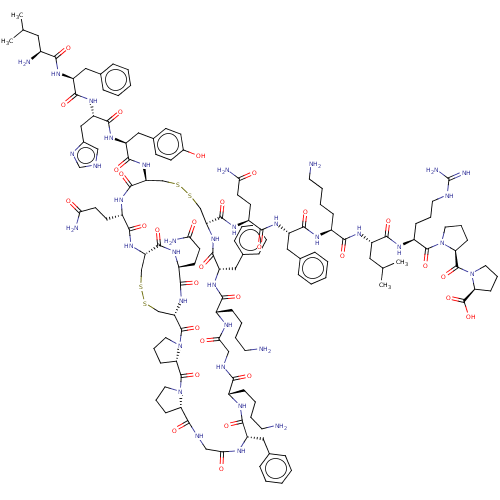
(CHEMBL5192562)Show SMILES [H][C@@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc3ccccc3)NC(=O)CNC2=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2CCC[C@H]2C(=O)N2CCC[C@H]2C(O)=O)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](N)CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00786
BindingDB Entry DOI: 10.7270/Q2PV6QBW |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50061562

((12R,13aR)-12-Methoxy-1,4,5,6,9,11,12,13-octahydro...)Show SMILES CO[C@@H]1CC=C2CCN3CCC4=C(CC(=O)OC4)[C@@]23C1 |t:4,11| Show InChI InChI=1S/C16H21NO3/c1-19-13-3-2-12-5-7-17-6-4-11-10-20-15(18)8-14(11)16(12,17)9-13/h2,13H,3-10H2,1H3/t13-,16-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by PDSP Ki Database
| |
Mol Pharmacol 54: 322-33 (1998)
Article DOI: 10.1124/mol.54.2.322
BindingDB Entry DOI: 10.7270/Q2ZW1JGJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data