| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C9 |
|---|
| Ligand | BDBM50425780 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_937208 (CHEMBL2318534) |
|---|
| IC50 | 110±n/a nM |
|---|
| Citation |  Ren, X; Pan, X; Zhang, Z; Wang, D; Lu, X; Li, Y; Wen, D; Long, H; Luo, J; Feng, Y; Zhuang, X; Zhang, F; Liu, J; Leng, F; Lang, X; Bai, Y; She, M; Tu, Z; Pan, J; Ding, K Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem56:879-94 (2013) [PubMed] Article Ren, X; Pan, X; Zhang, Z; Wang, D; Lu, X; Li, Y; Wen, D; Long, H; Luo, J; Feng, Y; Zhuang, X; Zhang, F; Liu, J; Leng, F; Lang, X; Bai, Y; She, M; Tu, Z; Pan, J; Ding, K Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem56:879-94 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C9 |
|---|
| Name: | Cytochrome P450 2C9 |
|---|
| Synonyms: | (R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55636.33 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11712 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKV
YGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKW
KEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICS
IIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFM
KSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTE
TTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYID
LLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFK
KSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVP
PFYQLCFIPV
|
|
|
|---|
| BDBM50425780 |
|---|
| n/a |
|---|
| Name | BDBM50425780 |
|---|
| Synonyms: | CHEMBL2316582 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H27F3N6O |
|---|
| Mol. Mass. | 532.5595 |
|---|
| SMILES | CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4[nH]ncc4c3)cc2C(F)(F)F)CC1 |
|---|
| Structure | 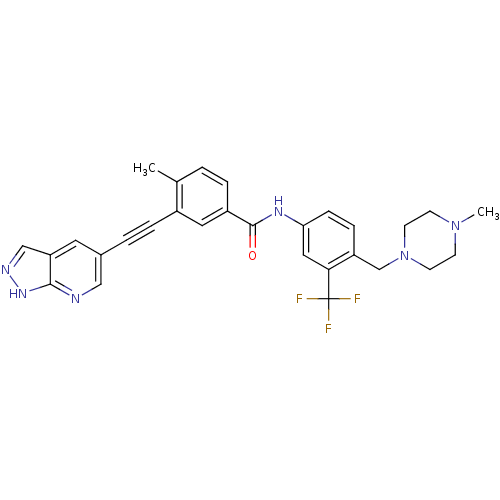
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ren, X; Pan, X; Zhang, Z; Wang, D; Lu, X; Li, Y; Wen, D; Long, H; Luo, J; Feng, Y; Zhuang, X; Zhang, F; Liu, J; Leng, F; Lang, X; Bai, Y; She, M; Tu, Z; Pan, J; Ding, K Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem56:879-94 (2013) [PubMed] Article
Ren, X; Pan, X; Zhang, Z; Wang, D; Lu, X; Li, Y; Wen, D; Long, H; Luo, J; Feng, Y; Zhuang, X; Zhang, F; Liu, J; Leng, F; Lang, X; Bai, Y; She, M; Tu, Z; Pan, J; Ding, K Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem56:879-94 (2013) [PubMed] Article