| Citation |  Clark, MP; Ledeboer, MW; Davies, I; Byrn, RA; Jones, SM; Perola, E; Tsai, A; Jacobs, M; Nti-Addae, K; Bandarage, UK; Boyd, MJ; Bethiel, RS; Court, JJ; Deng, H; Duffy, JP; Dorsch, WA; Farmer, LJ; Gao, H; Gu, W; Jackson, K; Jacobs, DH; Kennedy, JM; Ledford, B; Liang, J; Maltais, F; Murcko, M; Wang, T; Wannamaker, MW; Bennett, HB; Leeman, JR; McNeil, C; Taylor, WP; Memmott, C; Jiang, M; Rijnbrand, R; Bral, C; Germann, U; Nezami, A; Zhang, Y; Salituro, FG; Bennani, YL; Charifson, PS Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem57:6668-78 (2014) [PubMed] Article Clark, MP; Ledeboer, MW; Davies, I; Byrn, RA; Jones, SM; Perola, E; Tsai, A; Jacobs, M; Nti-Addae, K; Bandarage, UK; Boyd, MJ; Bethiel, RS; Court, JJ; Deng, H; Duffy, JP; Dorsch, WA; Farmer, LJ; Gao, H; Gu, W; Jackson, K; Jacobs, DH; Kennedy, JM; Ledford, B; Liang, J; Maltais, F; Murcko, M; Wang, T; Wannamaker, MW; Bennett, HB; Leeman, JR; McNeil, C; Taylor, WP; Memmott, C; Jiang, M; Rijnbrand, R; Bral, C; Germann, U; Nezami, A; Zhang, Y; Salituro, FG; Bennani, YL; Charifson, PS Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem57:6668-78 (2014) [PubMed] Article |
|---|
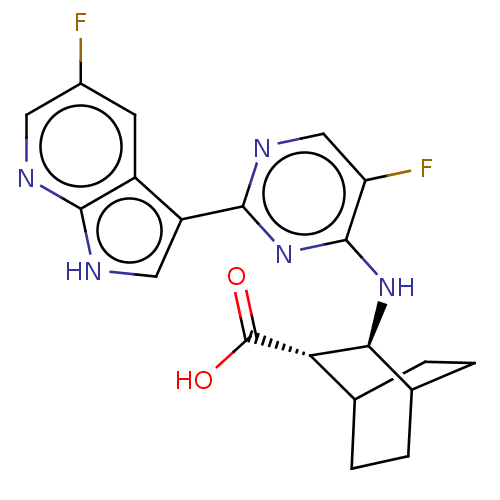
| SMILES | OC(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:3.2,wD:10.12,(15.61,-25.66,;14.11,-26.02,;13.05,-24.9,;13.67,-27.49,;14.73,-28.62,;14.29,-30.09,;12.8,-30.45,;11.74,-29.34,;12.83,-28.23,;13.22,-29.72,;12.18,-27.86,;11.12,-26.75,;9.63,-27.1,;9.19,-28.58,;7.69,-28.94,;6.63,-27.82,;7.06,-26.35,;8.56,-25.99,;9,-24.51,;7.25,-30.41,;8.2,-31.63,;7.32,-32.91,;5.84,-32.47,;4.53,-33.27,;3.18,-32.54,;3.14,-31,;1.79,-30.27,;4.45,-30.19,;5.8,-30.93,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Clark, MP; Ledeboer, MW; Davies, I; Byrn, RA; Jones, SM; Perola, E; Tsai, A; Jacobs, M; Nti-Addae, K; Bandarage, UK; Boyd, MJ; Bethiel, RS; Court, JJ; Deng, H; Duffy, JP; Dorsch, WA; Farmer, LJ; Gao, H; Gu, W; Jackson, K; Jacobs, DH; Kennedy, JM; Ledford, B; Liang, J; Maltais, F; Murcko, M; Wang, T; Wannamaker, MW; Bennett, HB; Leeman, JR; McNeil, C; Taylor, WP; Memmott, C; Jiang, M; Rijnbrand, R; Bral, C; Germann, U; Nezami, A; Zhang, Y; Salituro, FG; Bennani, YL; Charifson, PS Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem57:6668-78 (2014) [PubMed] Article
Clark, MP; Ledeboer, MW; Davies, I; Byrn, RA; Jones, SM; Perola, E; Tsai, A; Jacobs, M; Nti-Addae, K; Bandarage, UK; Boyd, MJ; Bethiel, RS; Court, JJ; Deng, H; Duffy, JP; Dorsch, WA; Farmer, LJ; Gao, H; Gu, W; Jackson, K; Jacobs, DH; Kennedy, JM; Ledford, B; Liang, J; Maltais, F; Murcko, M; Wang, T; Wannamaker, MW; Bennett, HB; Leeman, JR; McNeil, C; Taylor, WP; Memmott, C; Jiang, M; Rijnbrand, R; Bral, C; Germann, U; Nezami, A; Zhang, Y; Salituro, FG; Bennani, YL; Charifson, PS Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem57:6668-78 (2014) [PubMed] Article