| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Neuraminidase |
|---|
| Ligand | BDBM5025 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1449777 (CHEMBL3378764) |
|---|
| IC50 | 1.4±n/a nM |
|---|
| Citation |  Rakers, C; Schwerdtfeger, SM; Mortier, J; Duwe, S; Wolff, T; Wolber, G; Melzig, MF Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg Med Chem Lett24:4312-7 (2014) [PubMed] Article Rakers, C; Schwerdtfeger, SM; Mortier, J; Duwe, S; Wolff, T; Wolber, G; Melzig, MF Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg Med Chem Lett24:4312-7 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Neuraminidase |
|---|
| Name: | Neuraminidase |
|---|
| Synonyms: | NA | NRAM_I77AB | Neuraminidase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 51863.92 |
|---|
| Organism: | Influenza A virus (strain A/USSR/90/1977 H1N1) |
|---|
| Description: | ChEMBL_109720 |
|---|
| Residue: | 470 |
|---|
| Sequence: | MNPNQKIITIGSICMAIGIISLILQIGNIISIWVSHSIQTGSQNHTGICNQRIITYENST
WVNQTYVNISNTNVVAGKDTTSMTLAGNSSLCPIRGWAIYSKDNSIRIGSKGDVFVIREP
FISCSHLECRTFFLTQGALLNDKHSNGTVKDRSPYRALMSCPIGEAPSPYNSRFESVAWS
ASACHDGMGWLTIGISGPDDGAVAVLKYNGIITETIKSWRKQILRTQESECVCVNGSCFT
IMTDGPSDGPASYRIFKIEKGKITKSIELDAPNSHYEECSCYPDTGTVMCVCRDNWHGSN
RPWVSFNQNLDYQIGYICSGVFGDNPRPKDGKGSCDPVNVDGADGVKGFSYRYGNGVWIG
RTKSNSSRKGFEMIWDPNGWTDTDSNFLVKQDVVAMTDWSGYSGSFVQHPELTGLDCMRP
CFWVELIRGRPREKTTIWTSGSSISFCGVNSDTVNWSWPDGAELPFTIDK
|
|
|
|---|
| BDBM5025 |
|---|
| n/a |
|---|
| Name | BDBM5025 |
|---|
| Synonyms: | Oseltamivir | US10919856, POSITIVE CONTROL | ethyl (3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H28N2O4 |
|---|
| Mol. Mass. | 312.4045 |
|---|
| SMILES | [H][C@@]1(OC(CC)CC)C=C(C[C@H](N)[C@H]1NC(C)=O)C(=O)OCC |r,c:8| |
|---|
| Structure | 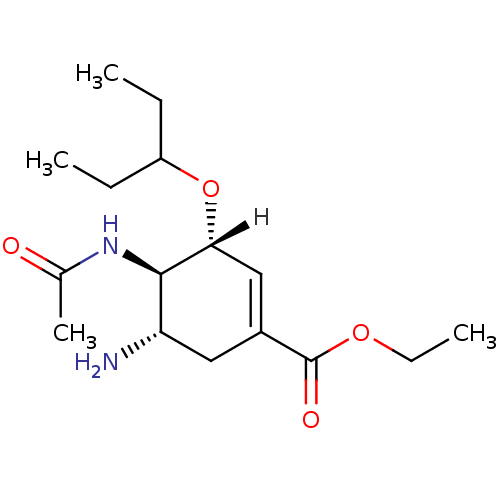
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Rakers, C; Schwerdtfeger, SM; Mortier, J; Duwe, S; Wolff, T; Wolber, G; Melzig, MF Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg Med Chem Lett24:4312-7 (2014) [PubMed] Article
Rakers, C; Schwerdtfeger, SM; Mortier, J; Duwe, S; Wolff, T; Wolber, G; Melzig, MF Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg Med Chem Lett24:4312-7 (2014) [PubMed] Article