

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120854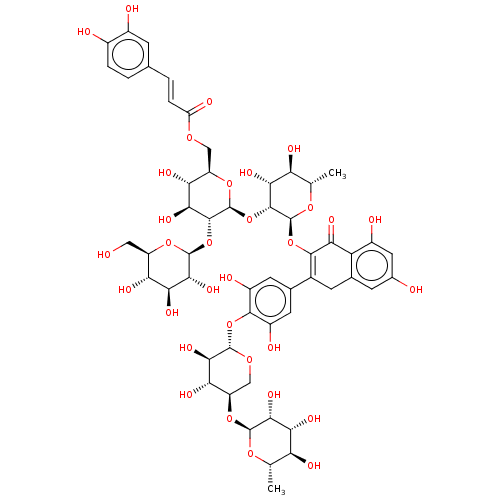 (CHEMBL3618493) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Competitive inhibition of human pancreatic alpha-amylase by double reciprocal plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120851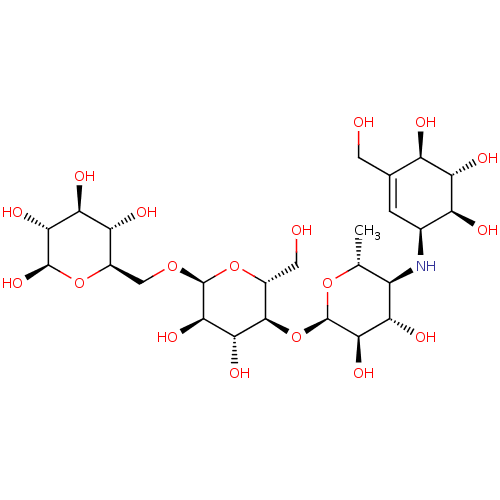 (CHEMBL1233515) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120850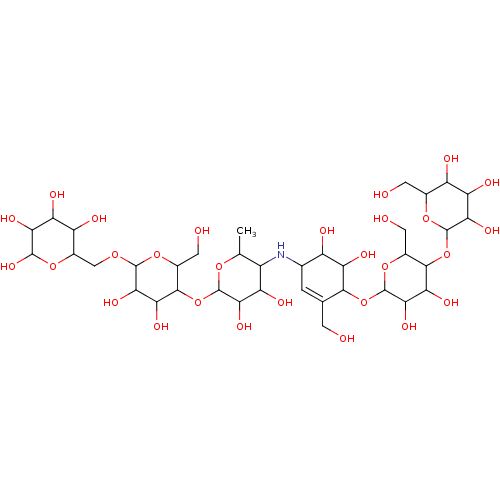 (CHEMBL3618491) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120843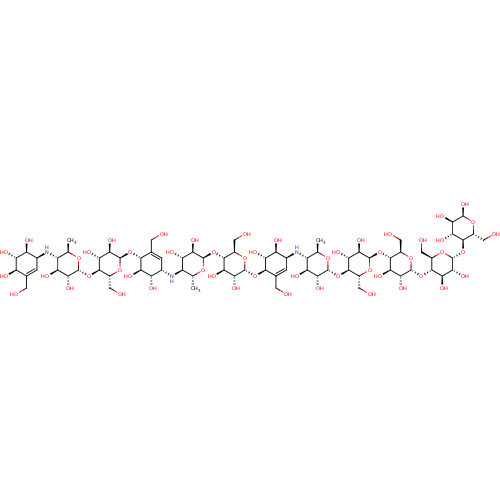 (CHEMBL3616594) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120842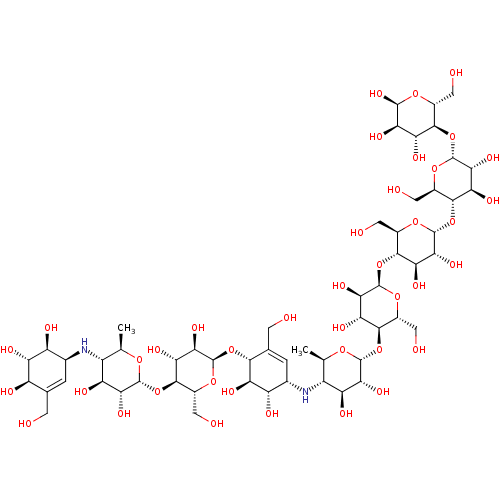 (CHEMBL3618489) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120844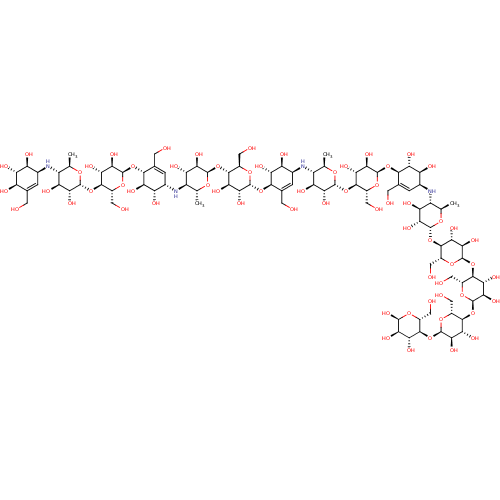 (CHEMBL3616595) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120852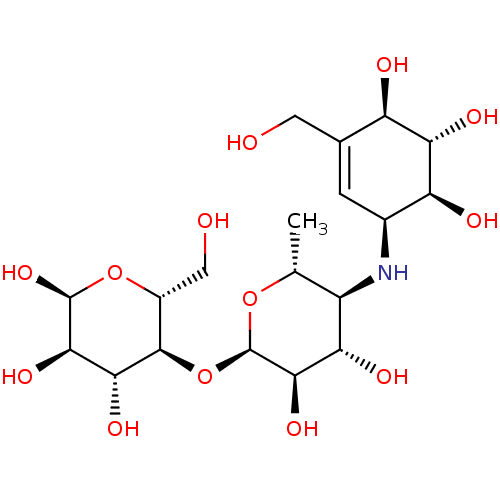 (CHEMBL1230193) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120853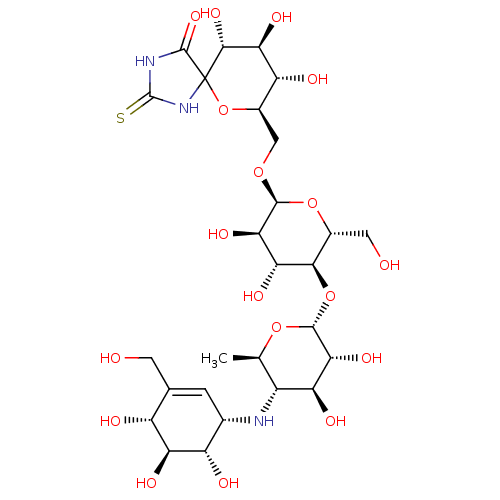 (CHEMBL3618492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Non-competitive inhibition of human salivary alpha-amylase using GalG2CNP as substrate assessed as CNP liberation by Lineweaver-Burk plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120849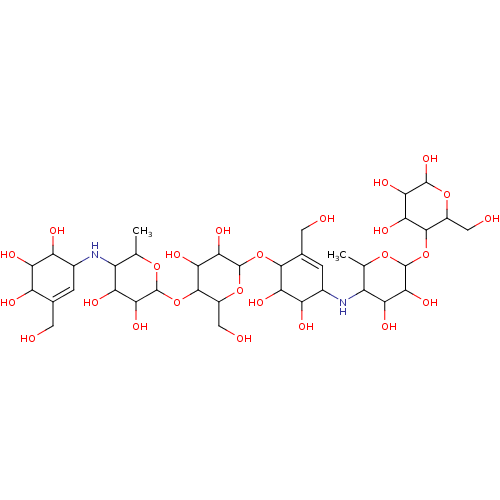 (CHEMBL3618490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120848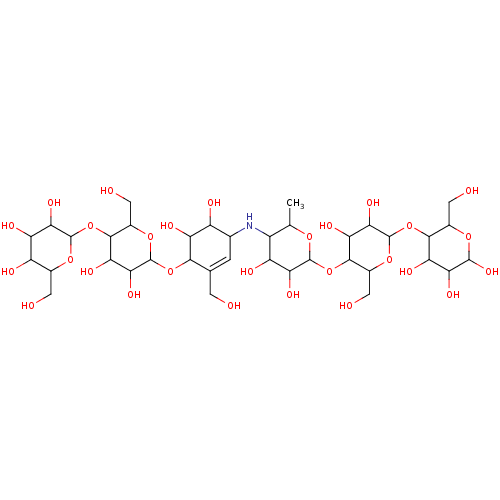 (CHEMBL3618488) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50241052 (1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Non-competitive inhibition of human salivary alpha-amylase using GalG2CNP as substrate assessed as CNP liberation by Lineweaver-Burk plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120855 (CHEMBL3618494) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Competitive inhibition of human pancreatic alpha-amylase by double reciprocal plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120856 (CHEMBL3618495) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Competitive inhibition of human pancreatic alpha-amylase by double reciprocal plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120839 (CHEMBL1234040) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase assessed as hydrolysis of G3F by Dixon plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM29143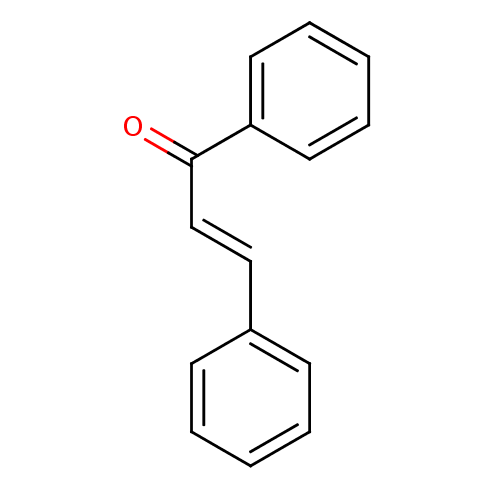 (CHEMBL7976 | Chalcone 1 | Chalcone, 13 | cid_63776...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha-amylase using soluble starch as substrate after 30 mins by Bernfeld method | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120838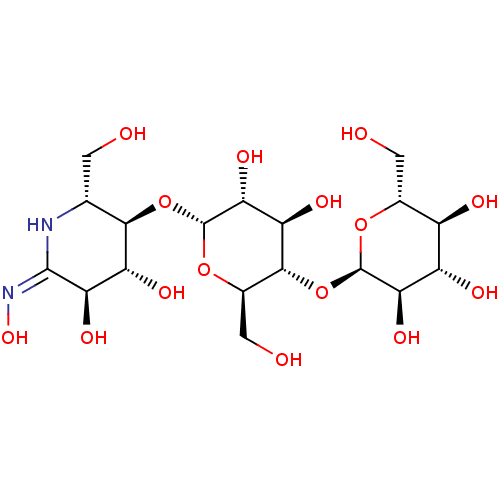 (CHEMBL1233953) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase assessed as hydrolysis of G3F by Dixon plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase type A isozyme (Hordeum vulgare) | BDBM50120841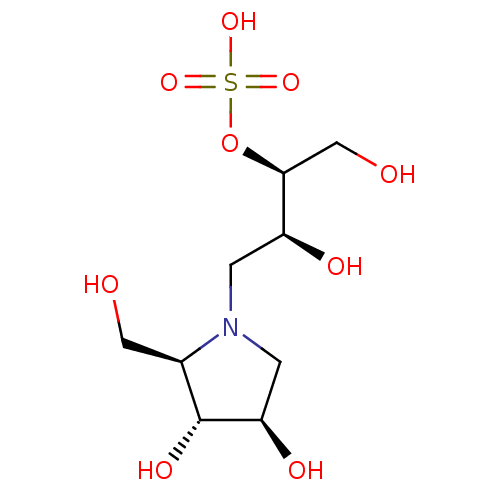 (CHEMBL3616593) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of recombinant barley alpha-amylase isozyme-1 using DP17 amylose as substrate preincubated for 5 mins followed by substrate addition by co... | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50120841 (CHEMBL3616593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic alpha-amylase using DP17 amylose as substrate preincubated for 5 mins followed by substrate addition by copper-bicin... | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120840 (CHEMBL1213470) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase assessed as hydrolysis of G3F by Dixon plot analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50333465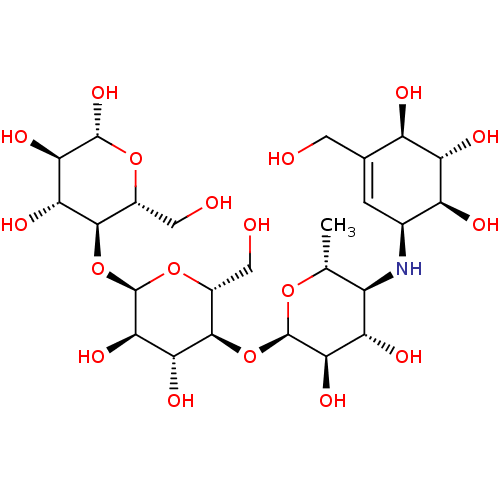 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50241052 (1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate assessed as CNP liberation by spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Homo sapiens (Human)) | BDBM50120847 (CHEMBL3618487) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120845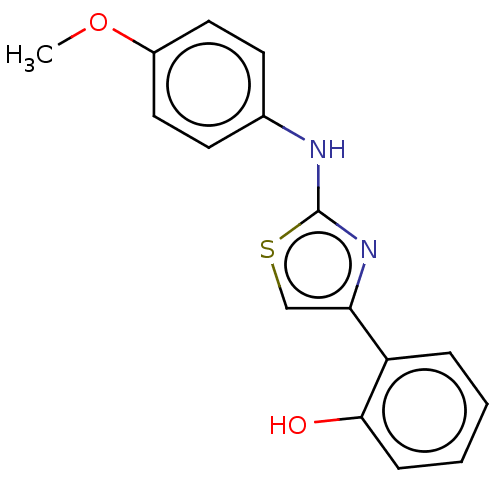 (CHEMBL3618485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120834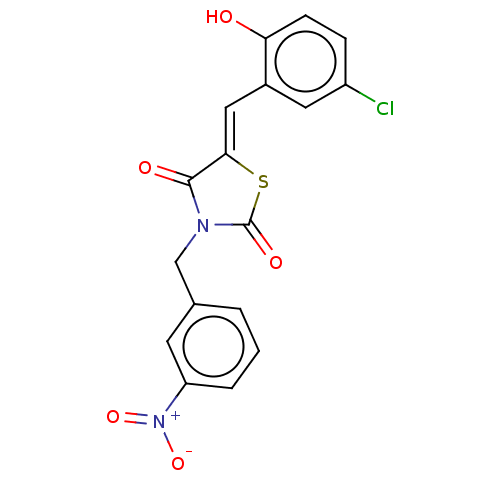 (CHEMBL2011396) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM32252 (2-(1-benzimidazolyl)-1-(5-ethyl-2,4-dihydroxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of HSP90 (unknown origin) | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50241242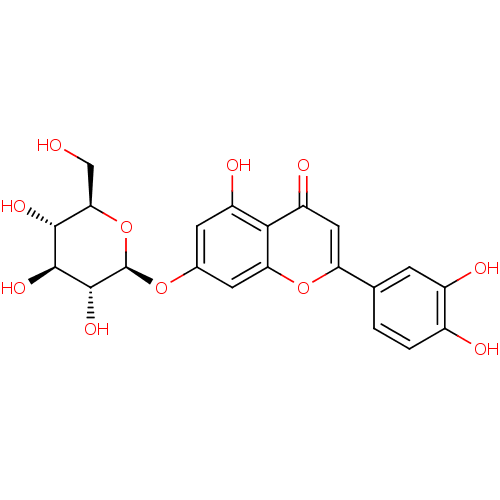 (2-(3,4-dihydroxyphenyl)-5-hydroxy-4-oxo-4H-chromen...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120835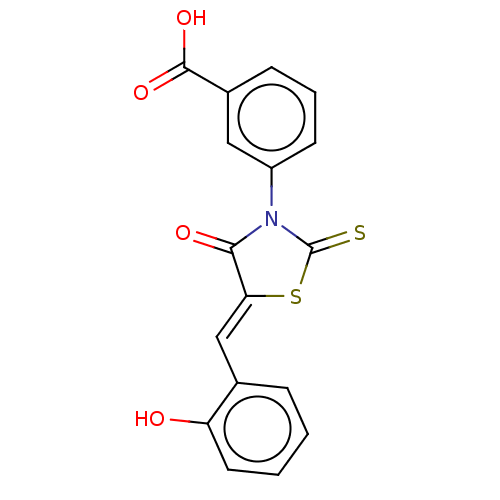 (CHEMBL2011401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50241367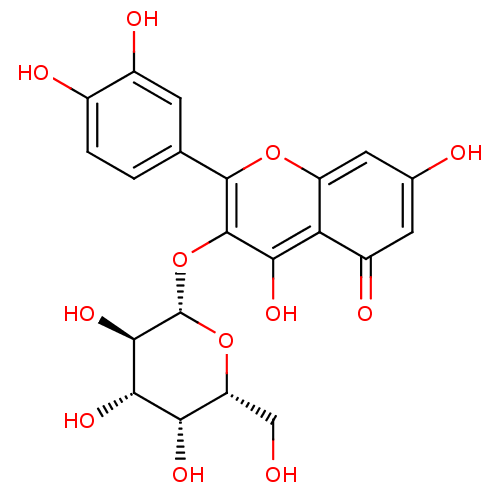 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-((2S,4R,5...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50241242 (2-(3,4-dihydroxyphenyl)-5-hydroxy-4-oxo-4H-chromen...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50241367 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-((2S,4R,5...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50217942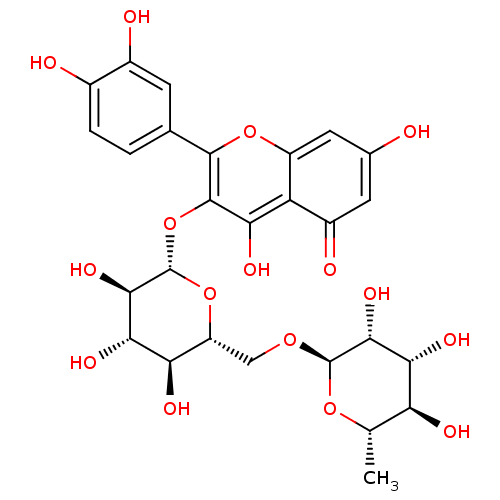 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50217942 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120832 (CHEMBL3618483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120831 (CHEMBL3618482) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50056315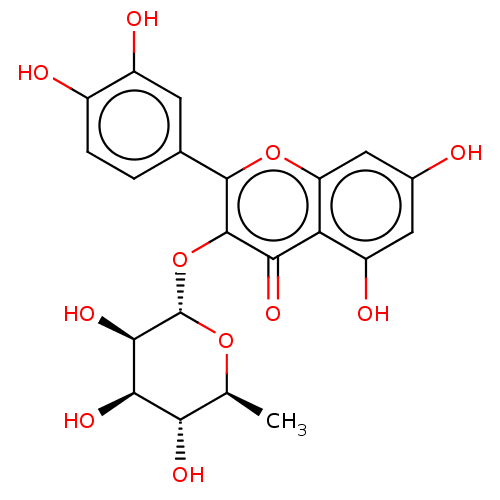 (CHEBI:17558 | Quercitrin) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50056315 (CHEBI:17558 | Quercitrin) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50293578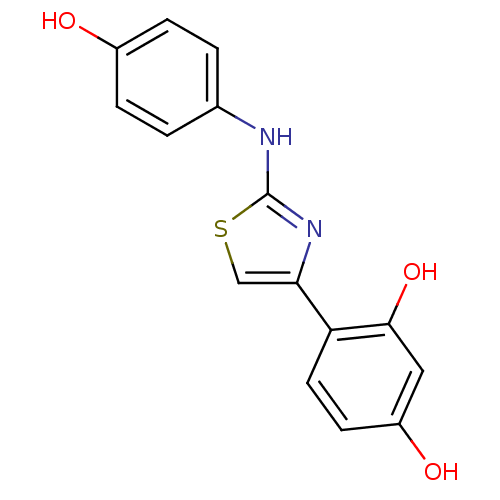 (4-(2-(4-Hydroxyphenylamino)thiazol-4-yl)benzene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50293573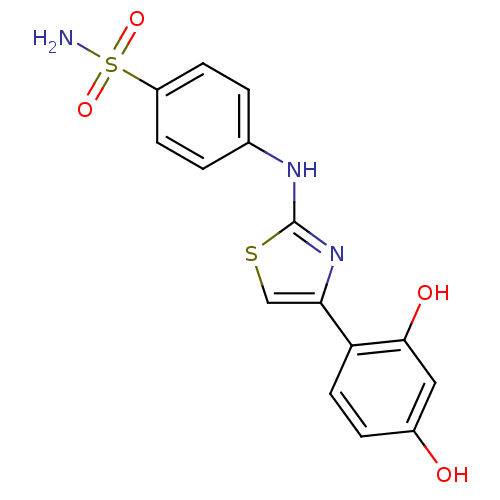 (4-(4-(2,4-dihydroxyphenyl)thiazol-2-ylamino)benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120823 (CHEMBL1904421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120836 (CHEMBL572150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120846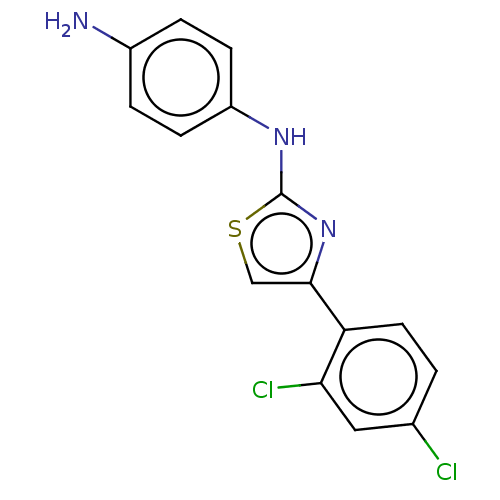 (CHEMBL3618486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120824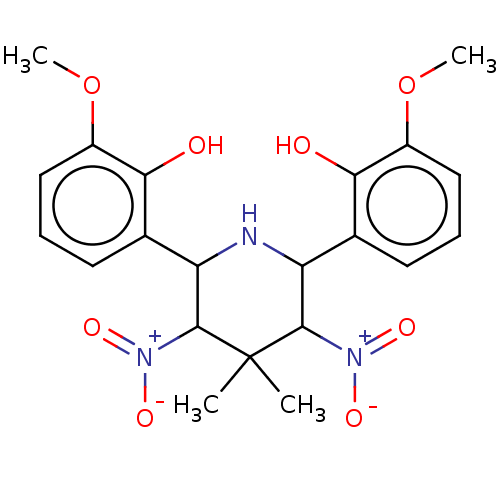 (CHEMBL3618478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120826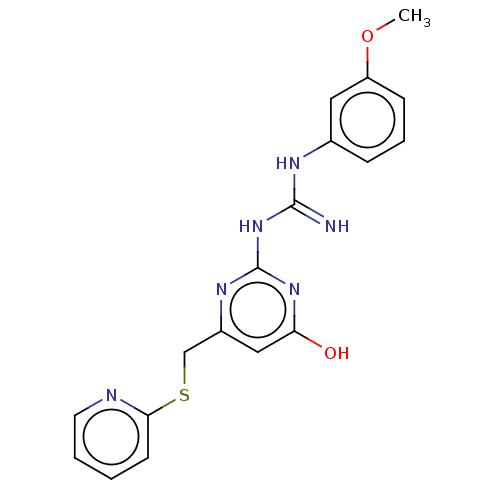 (CHEMBL1510984) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50241354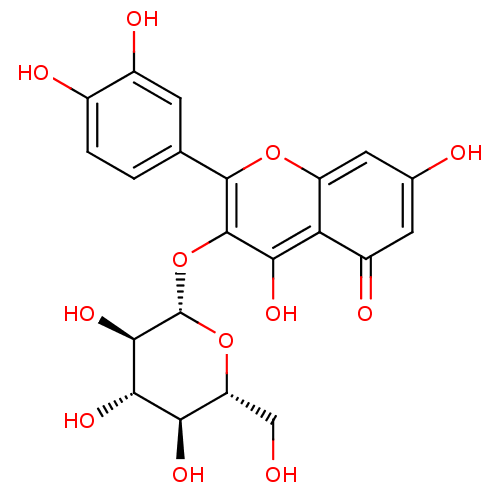 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-amylase 1A (Homo sapiens (Human)) | BDBM50120830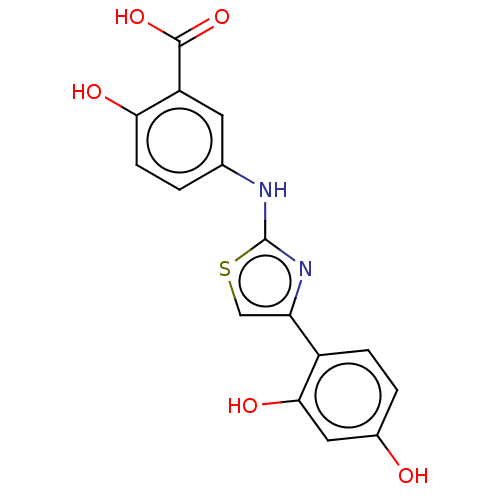 (CHEMBL599385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Inhibition of human salivary alpha-amylase using GalG2CNP as substrate by UV-Vis spectrophotometric analysis | Bioorg Med Chem 23: 6725-32 (2015) Article DOI: 10.1016/j.bmc.2015.09.007 BindingDB Entry DOI: 10.7270/Q21G0P2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50241354 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin Curated by ChEMBL | Assay Description Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... | Bioorg Med Chem Lett 24: 4312-7 (2014) Article DOI: 10.1016/j.bmcl.2014.07.010 BindingDB Entry DOI: 10.7270/Q2BC4164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |