 Found 1249 hits with Last Name = 'mortier' and Initial = 'j'
Found 1249 hits with Last Name = 'mortier' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120854
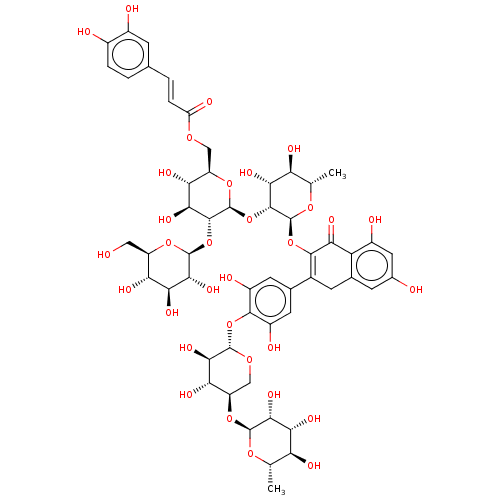
(CHEMBL3618493)Show SMILES C[C@@H]1O[C@@H](O[C@@H]2CO[C@@H](Oc3c(O)cc(cc3O)C3=C(O[C@@H]4O[C@@H](C)[C@H](O)[C@@H](O)[C@H]4O[C@@H]4O[C@H](COC(=O)\C=C\c5ccc(O)c(O)c5)[C@@H](O)[C@H](O)[C@H]4O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)C(=O)c4c(O)cc(O)cc4C3)[C@H](O)[C@H]2O)[C@H](O)[C@H](O)[C@H]1O |r,c:19| Show InChI InChI=1S/C54H66O32/c1-16-33(63)39(69)44(74)51(78-16)81-30-15-77-50(43(73)37(30)67)84-47-26(60)10-19(11-27(47)61)22-9-20-8-21(56)12-25(59)32(20)38(68)46(22)83-53-48(41(71)34(64)17(2)79-53)86-54-49(85-52-45(75)40(70)35(65)28(13-55)80-52)42(72)36(66)29(82-54)14-76-31(62)6-4-18-3-5-23(57)24(58)7-18/h3-8,10-12,16-17,28-30,33-37,39-45,48-61,63-67,69-75H,9,13-15H2,1-2H3/b6-4+/t16-,17-,28+,29+,30+,33-,34-,35+,36+,37-,39+,40-,41+,42-,43+,44+,45+,48+,49+,50-,51-,52-,53-,54-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Competitive inhibition of human pancreatic alpha-amylase by double reciprocal plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120851
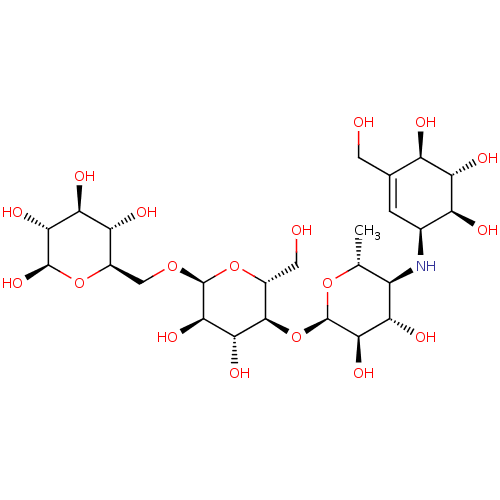
(CHEMBL1233515)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](OC[C@H]3O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |r,t:37| Show InChI InChI=1S/C25H43NO18/c1-6-11(26-8-2-7(3-27)12(29)16(33)13(8)30)15(32)20(37)25(41-6)44-22-9(4-28)43-24(21(38)18(22)35)40-5-10-14(31)17(34)19(36)23(39)42-10/h2,6,8-39H,3-5H2,1H3/t6-,8+,9-,10-,11-,12-,13+,14-,15+,16+,17+,18-,19-,20-,21-,22-,23-,24+,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120850
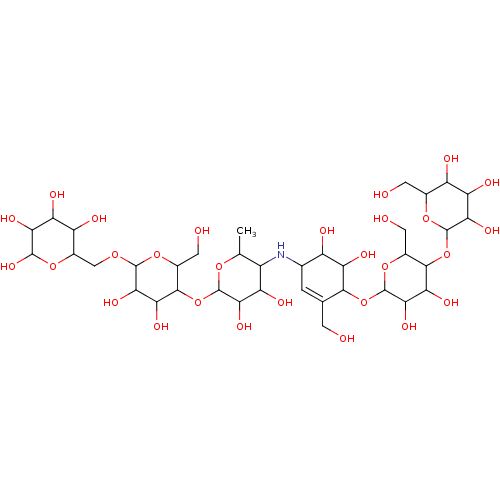
(CHEMBL3618491)Show SMILES CC1OC(OC2C(CO)OC(OCC3OC(O)C(O)C(O)C3O)C(O)C2O)C(O)C(O)C1NC1C=C(CO)C(OC2OC(CO)C(OC3OC(CO)C(O)C(O)C3O)C(O)C2O)C(O)C1O |t:37| Show InChI InChI=1S/C37H63NO28/c1-8-15(19(46)26(53)35(59-8)65-31-12(5-41)62-34(28(55)23(31)50)58-7-14-18(45)20(47)25(52)33(57)60-14)38-10-2-9(3-39)30(22(49)16(10)43)64-37-29(56)24(51)32(13(6-42)63-37)66-36-27(54)21(48)17(44)11(4-40)61-36/h2,8,10-57H,3-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120843
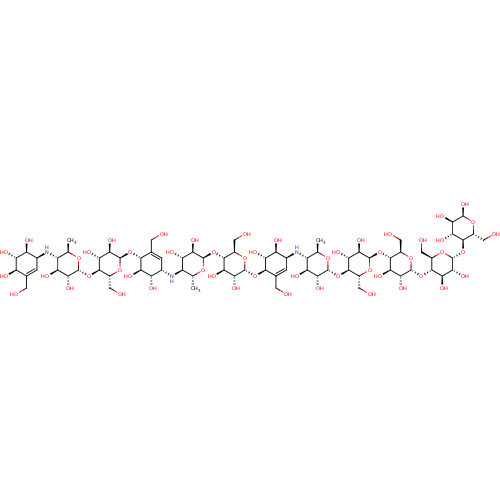
(CHEMBL3616594)Show SMILES [H][C@]1(O[C@@H]2[C@@H](CO)O[C@H](O[C@H]3[C@H](O)[C@@H](O)[C@@H](N[C@@H]4[C@@H](C)O[C@]([H])(O[C@@H]5[C@@H](CO)O[C@H](O[C@]6([H])[C@@H](CO)O[C@H](O[C@]7([H])[C@@H](CO)O[C@H](O[C@]8([H])[C@@H](CO)O[C@H](O)[C@H](O)[C@H]8O)[C@H](O)[C@H]7O)[C@H](O)[C@H]6O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)C=C3CO)[C@H](O)[C@H]2O)O[C@H](C)[C@@H](N[C@H]2C=C(CO)[C@@]([H])(O[C@H]3O[C@H](CO)[C@@H](O[C@@]4([H])O[C@H](C)[C@@H](N[C@H]5C=C(CO)[C@@H](O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r,c:80,t:96,118| Show InChI InChI=1S/C75H125N3O52/c1-16-31(76-22-4-19(7-79)34(88)41(95)35(22)89)38(92)51(105)68(114-16)125-62-26(11-83)118-71(54(108)45(62)99)123-59-20(8-80)5-23(36(90)42(59)96)77-32-17(2)115-69(52(106)39(32)93)126-63-27(12-84)119-72(55(109)46(63)100)124-60-21(9-81)6-24(37(91)43(60)97)78-33-18(3)116-70(53(107)40(33)94)127-64-28(13-85)120-74(56(110)47(64)101)129-66-30(15-87)122-75(58(112)49(66)103)130-65-29(14-86)121-73(57(111)48(65)102)128-61-25(10-82)117-67(113)50(104)44(61)98/h4-6,16-18,22-113H,7-15H2,1-3H3/t16-,17-,18-,22+,23+,24+,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35+,36+,37+,38+,39+,40+,41+,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67+,68-,69-,70-,71-,72-,73-,74-,75-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120842
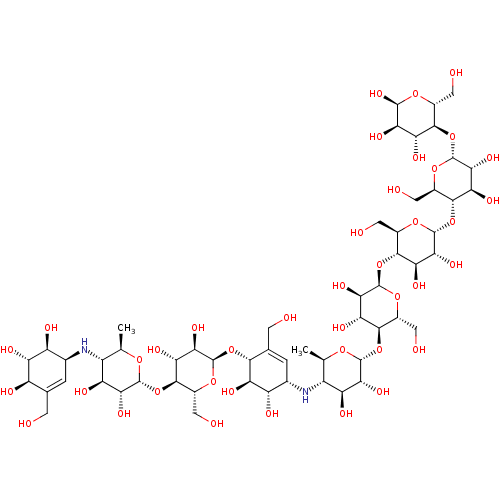
(CHEMBL3618489)Show SMILES [H][C@]1(O[C@@H]2[C@@H](CO)O[C@H](O[C@]3([H])[C@@H](CO)O[C@H](O[C@]4([H])[C@@H](CO)O[C@H](O[C@]5([H])[C@@H](CO)O[C@H](O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)O[C@H](C)[C@@H](N[C@H]2C=C(CO)[C@@H](O[C@@]3([H])O[C@H](CO)[C@@H](O[C@@]4([H])O[C@H](C)[C@@H](N[C@H]5C=C(CO)[C@@H](O)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r,t:60,82| Show InChI InChI=1S/C56H94N2O40/c1-12-23(57-16-3-14(5-59)25(66)30(71)26(16)67)28(69)38(79)51(86-12)94-46-19(8-62)89-53(40(81)33(46)74)93-44-15(6-60)4-17(27(68)31(44)72)58-24-13(2)87-52(39(80)29(24)70)95-47-20(9-63)90-55(41(82)34(47)75)97-49-22(11-65)92-56(43(84)36(49)77)98-48-21(10-64)91-54(42(83)35(48)76)96-45-18(7-61)88-50(85)37(78)32(45)73/h3-4,12-13,16-85H,5-11H2,1-2H3/t12-,13-,16+,17+,18-,19-,20-,21-,22-,23-,24-,25-,26+,27+,28+,29+,30+,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50+,51-,52-,53-,54-,55-,56-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120844
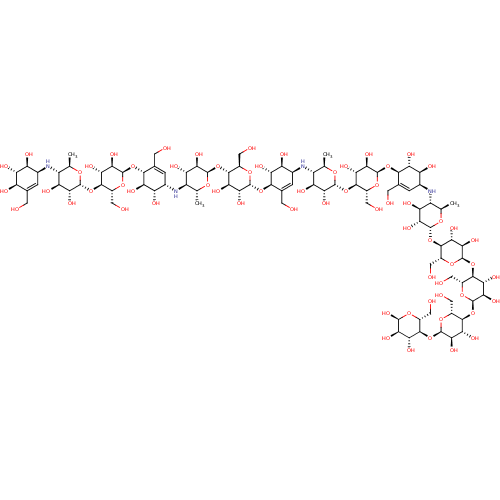
(CHEMBL3616595)Show SMILES [H][C@]1(O[C@@H]2[C@@H](CO)O[C@H](O[C@H]3[C@H](O)[C@@H](O)[C@@H](N[C@@H]4[C@@H](C)O[C@]([H])(O[C@@H]5[C@@H](CO)O[C@H](O[C@@]6([H])[C@H](O)[C@@H](O)[C@@H](N[C@@H]7[C@@H](C)O[C@]([H])(O[C@@H]8[C@@H](CO)O[C@H](O[C@H]9[C@H](O)[C@@H](O)[C@@H](N[C@@H]%10[C@@H](C)O[C@]([H])(O[C@@H]%11[C@@H](CO)O[C@H](O[C@]%12([H])[C@@H](CO)O[C@H](O[C@]%13([H])[C@@H](CO)O[C@H](O[C@]%14([H])[C@@H](CO)O[C@H](O)[C@H](O)[C@H]%14O)[C@H](O)[C@H]%13O)[C@H](O)[C@H]%12O)[C@H](O)[C@H]%11O)[C@H](O)[C@H]%10O)C=C9CO)[C@H](O)[C@H]8O)[C@H](O)[C@H]7O)C=C6CO)[C@H](O)[C@H]5O)[C@H](O)[C@H]4O)C=C3CO)[C@H](O)[C@H]2O)O[C@H](C)[C@@H](N[C@H]2C=C(CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r,c:123,138,153,t:169| Show InChI InChI=1S/C94H156N4O64/c1-20-39(95-28-5-24(9-99)43(110)52(119)44(28)111)48(115)64(131)85(142-20)156-78-33(14-104)147-89(68(135)57(78)124)153-74-25(10-100)6-29(45(112)53(74)120)96-40-21(2)143-86(65(132)49(40)116)157-79-34(15-105)148-90(69(136)58(79)125)154-75-26(11-101)7-30(46(113)54(75)121)97-41-22(3)144-87(66(133)50(41)117)158-80-35(16-106)149-91(70(137)59(80)126)155-76-27(12-102)8-31(47(114)55(76)122)98-42-23(4)145-88(67(134)51(42)118)159-81-36(17-107)150-93(71(138)60(81)127)161-83-38(19-109)152-94(73(140)62(83)129)162-82-37(18-108)151-92(72(139)61(82)128)160-77-32(13-103)146-84(141)63(130)56(77)123/h5-8,20-23,28-141H,9-19H2,1-4H3/t20-,21-,22-,23-,28+,29+,30+,31+,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84+,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120852
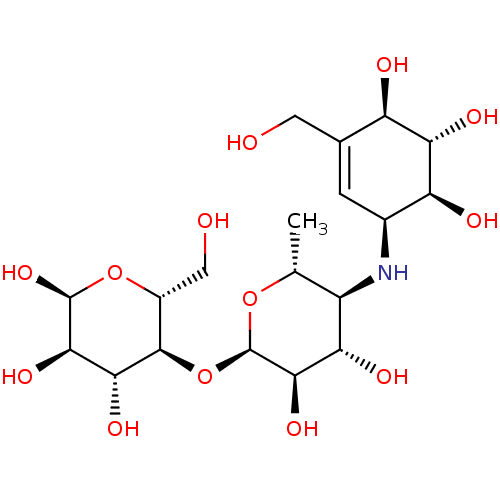
(CHEMBL1230193)Show SMILES C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |r,t:25| Show InChI InChI=1S/C19H33NO13/c1-5-9(20-7-2-6(3-21)10(23)13(26)11(7)24)12(25)16(29)19(31-5)33-17-8(4-22)32-18(30)15(28)14(17)27/h2,5,7-30H,3-4H2,1H3/t5-,7+,8-,9-,10-,11+,12+,13+,14-,15-,16-,17-,18+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-amylase 1A
(Homo sapiens (Human)) | BDBM50120853
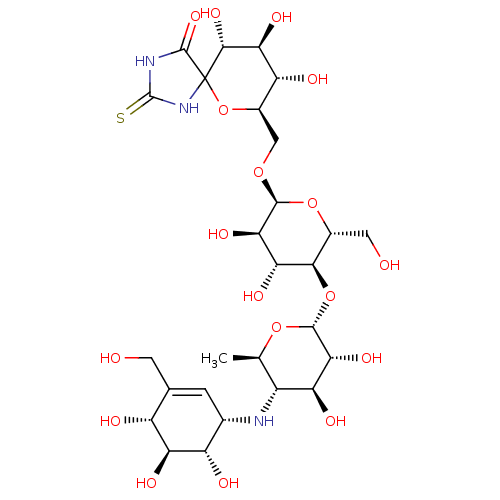
(CHEMBL3618492)Show SMILES [H][C@]1(O[C@H]2[C@H](O)[C@@H](O)[C@@H](OC[C@H]3OC4(NC(=S)NC4=O)[C@H](O)[C@@H](O)[C@@H]3O)O[C@@H]2CO)O[C@H](C)[C@@H](N[C@H]2C=C(CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r,t:39| Show InChI InChI=1S/C27H43N3O18S/c1-6-11(28-8-2-7(3-31)12(33)16(37)13(8)34)15(36)19(40)24(45-6)47-21-9(4-32)46-23(20(41)17(21)38)44-5-10-14(35)18(39)22(42)27(48-10)25(43)29-26(49)30-27/h2,6,8-24,28,31-42H,3-5H2,1H3,(H2,29,30,43,49)/t6-,8+,9-,10-,11-,12-,13+,14-,15+,16+,17-,18+,19-,20-,21-,22-,23+,24-,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human salivary alpha-amylase using GalG2CNP as substrate assessed as CNP liberation by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444695
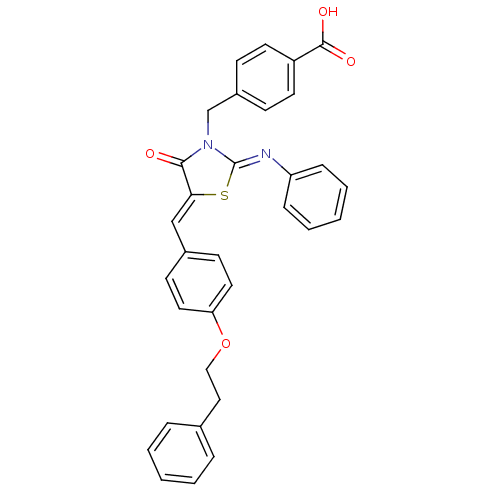
(CHEMBL3098852)Show SMILES OC(=O)c1ccc(CN2\C(S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)=N\c2ccccc2)cc1 Show InChI InChI=1S/C32H26N2O4S/c35-30-29(21-24-13-17-28(18-14-24)38-20-19-23-7-3-1-4-8-23)39-32(33-27-9-5-2-6-10-27)34(30)22-25-11-15-26(16-12-25)31(36)37/h1-18,21H,19-20,22H2,(H,36,37)/b29-21-,33-32- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Alpha-amylase 1A
(Homo sapiens (Human)) | BDBM50120849
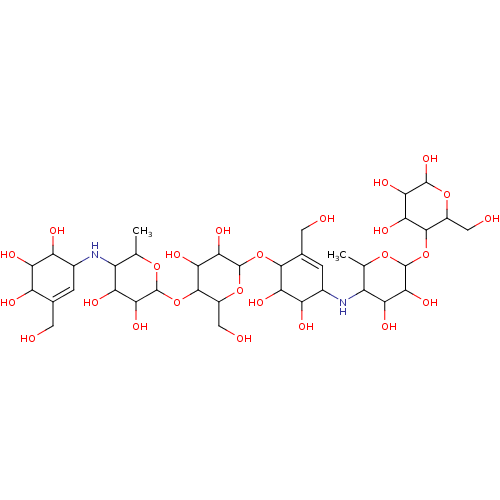
(CHEMBL3618490)Show SMILES CC1OC(OC2C(CO)OC(OC3C(O)C(O)C(NC4C(C)OC(OC5C(CO)OC(O)C(O)C5O)C(O)C4O)C=C3CO)C(O)C2O)C(O)C(O)C1NC1C=C(CO)C(O)C(O)C1O |c:42,t:60| Show InChI InChI=1S/C38H64N2O25/c1-9-17(39-13-3-11(5-41)19(45)24(50)20(13)46)22(48)30(56)37(60-9)65-34-16(8-44)62-38(31(57)27(34)53)63-32-12(6-42)4-14(21(47)25(32)51)40-18-10(2)59-36(29(55)23(18)49)64-33-15(7-43)61-35(58)28(54)26(33)52/h3-4,9-10,13-58H,5-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human salivary alpha-amylase using GalG2CNP as substrate |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120848
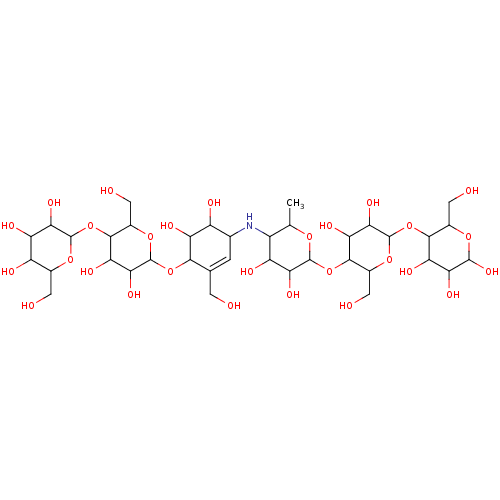
(CHEMBL3618488)Show SMILES CC1OC(OC2C(CO)OC(OC3C(CO)OC(O)C(O)C3O)C(O)C2O)C(O)C(O)C1NC1C=C(CO)C(OC2OC(CO)C(OC3OC(CO)C(O)C(O)C3O)C(O)C2O)C(O)C1O |t:37| Show InChI InChI=1S/C37H63NO28/c1-8-15(18(46)25(53)34(58-8)64-31-13(6-42)62-37(28(56)23(31)51)65-30-12(5-41)59-33(57)24(52)21(30)49)38-10-2-9(3-39)29(20(48)16(10)44)63-36-27(55)22(50)32(14(7-43)61-36)66-35-26(54)19(47)17(45)11(4-40)60-35/h2,8,10-57H,3-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase expressed in Pichia pastoris using amylase as substrate preincubated with substrate for 10 mins followed... |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444693
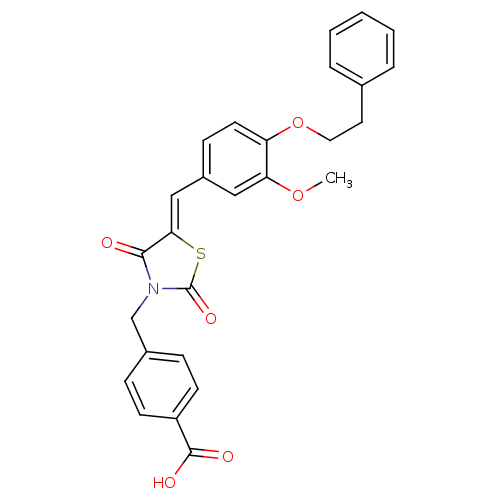
(CHEMBL3098944)Show SMILES COc1cc(\C=C2/SC(=O)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C27H23NO6S/c1-33-23-15-20(9-12-22(23)34-14-13-18-5-3-2-4-6-18)16-24-25(29)28(27(32)35-24)17-19-7-10-21(11-8-19)26(30)31/h2-12,15-16H,13-14,17H2,1H3,(H,30,31)/b24-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444694

(CHEMBL3098854)Show SMILES COc1cc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)ccc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-29-20-25(14-17-28(29)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444692

(CHEMBL3098853)Show SMILES COc1ccc(\C=C2/S\C(=N/c3ccccc3)N(Cc3ccc(cc3)C(O)=O)C2=O)cc1OCCc1ccccc1 Show InChI InChI=1S/C33H28N2O5S/c1-39-28-17-14-25(20-29(28)40-19-18-23-8-4-2-5-9-23)21-30-31(36)35(22-24-12-15-26(16-13-24)32(37)38)33(41-30)34-27-10-6-3-7-11-27/h2-17,20-21H,18-19,22H2,1H3,(H,37,38)/b30-21-,34-33- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal pl... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50444696
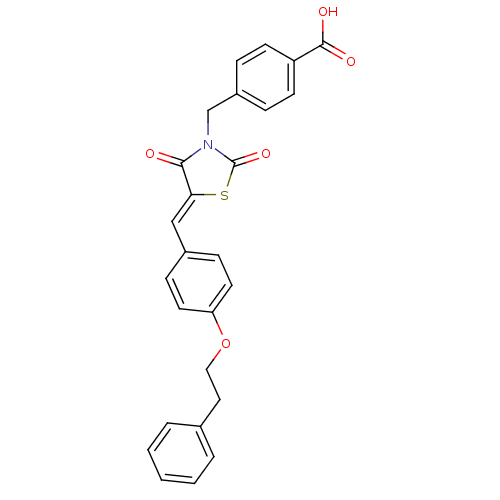
(CHEMBL3098942)Show SMILES OC(=O)c1ccc(CN2C(=O)S\C(=C/c3ccc(OCCc4ccccc4)cc3)C2=O)cc1 Show InChI InChI=1S/C26H21NO5S/c28-24-23(33-26(31)27(24)17-20-6-10-21(11-7-20)25(29)30)16-19-8-12-22(13-9-19)32-15-14-18-4-2-1-3-5-18/h1-13,16H,14-15,17H2,(H,29,30)/b23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human recombinant PTP1B expressed in Escherichia coli TB1 using p-nitrophenylphosphate as substrate by Double reciprocal plo... |
Eur J Med Chem 71: 112-27 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.001
BindingDB Entry DOI: 10.7270/Q2VH5Q95 |
More data for this
Ligand-Target Pair | |
Alpha-amylase 1A
(Homo sapiens (Human)) | BDBM50241052

(1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33-,34+,35-,41+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human salivary alpha-amylase using GalG2CNP as substrate assessed as CNP liberation by Lineweaver-Burk plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120855

(CHEMBL3618494)Show SMILES C[C@@H]1O[C@@H](O[C@@H]2CO[C@@H](Oc3c(O)cc(cc3O)C3=C(O[C@@H]4O[C@@H](C)[C@H](O)[C@@H](O)[C@H]4O[C@@H]4O[C@H](COC(=O)\C=C\c5ccc(O)cc5)[C@@H](O)[C@H](O)[C@H]4O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)C(=O)c4c(O)cc(O)cc4C3)[C@H](O)[C@H]2O)[C@H](O)[C@H](O)[C@H]1O |r,c:19| Show InChI InChI=1S/C54H66O31/c1-17-33(62)39(68)44(73)51(77-17)80-30-16-76-50(43(72)37(30)66)83-47-26(59)11-20(12-27(47)60)24-10-21-9-23(57)13-25(58)32(21)38(67)46(24)82-53-48(41(70)34(63)18(2)78-53)85-54-49(84-52-45(74)40(69)35(64)28(14-55)79-52)42(71)36(65)29(81-54)15-75-31(61)8-5-19-3-6-22(56)7-4-19/h3-9,11-13,17-18,28-30,33-37,39-45,48-60,62-66,68-74H,10,14-16H2,1-2H3/b8-5+/t17-,18-,28+,29+,30+,33-,34-,35+,36+,37-,39+,40-,41+,42-,43+,44+,45+,48+,49+,50-,51-,52-,53-,54-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Competitive inhibition of human pancreatic alpha-amylase by double reciprocal plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120856

(CHEMBL3618495)Show SMILES COc1cc(\C=C\C(=O)OC[C@H]2O[C@@H](O[C@@H]3[C@H](O)[C@@H](O)[C@H](C)O[C@H]3OC3=C(Cc4cc(O)cc(O)c4C3=O)c3cc(O)c(O[C@@H]4OC[C@@H](O[C@@H]5O[C@@H](C)[C@H](O)[C@@H](O)[C@H]5O)[C@H](O)[C@H]4O)c(O)c3)[C@H](O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@@H](O)[C@@H]2O)ccc1O |r,t:26| Show InChI InChI=1S/C55H68O32/c1-17-34(63)40(69)45(74)52(79-17)82-31-16-78-51(44(73)38(31)67)85-48-26(60)11-20(12-27(48)61)23-10-21-9-22(57)13-25(59)33(21)39(68)47(23)84-54-49(42(71)35(64)18(2)80-54)87-55-50(86-53-46(75)41(70)36(65)29(14-56)81-53)43(72)37(66)30(83-55)15-77-32(62)7-5-19-4-6-24(58)28(8-19)76-3/h4-9,11-13,17-18,29-31,34-38,40-46,49-61,63-67,69-75H,10,14-16H2,1-3H3/b7-5+/t17-,18-,29+,30+,31+,34-,35-,36+,37+,38-,40+,41-,42+,43-,44+,45+,46+,49+,50+,51-,52-,53-,54-,55-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Competitive inhibition of human pancreatic alpha-amylase by double reciprocal plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120839

(CHEMBL1234040)Show SMILES CO[C@@H]1[C@@H](CO)O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)N\C(=N\O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H34N2O15/c1-32-15-6(3-23)33-19(12(29)9(15)26)36-16-7(4-24)34-18(13(30)10(16)27)35-14-5(2-22)20-17(21-31)11(28)8(14)25/h5-16,18-19,22-31H,2-4H2,1H3,(H,20,21)/t5-,6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16-,18-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase assessed as hydrolysis of G3F by Dixon plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Sus scrofa (Pig)) | BDBM29143
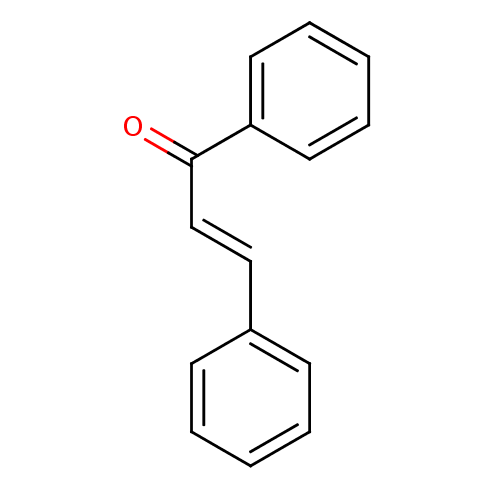
(CHEMBL7976 | Chalcone 1 | Chalcone, 13 | cid_63776...)Show InChI InChI=1S/C15H12O/c16-15(14-9-5-2-6-10-14)12-11-13-7-3-1-4-8-13/h1-12H/b12-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of porcine pancreatic alpha-amylase using soluble starch as substrate after 30 mins by Bernfeld method |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120838
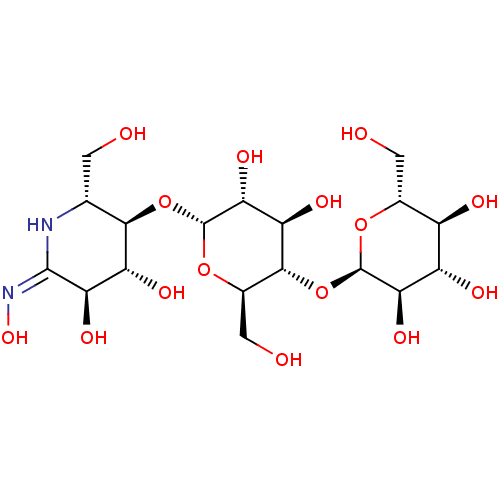
(CHEMBL1233953)Show SMILES OC[C@H]1N\C(=N\O)[C@H](O)[C@@H](O)[C@@H]1O[C@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H32N2O15/c21-1-4-14(9(26)11(28)16(19-4)20-31)34-18-13(30)10(27)15(6(3-23)33-18)35-17-12(29)8(25)7(24)5(2-22)32-17/h4-15,17-18,21-31H,1-3H2,(H,19,20)/t4-,5-,6-,7-,8+,9-,10-,11-,12-,13-,14-,15-,17-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase assessed as hydrolysis of G3F by Dixon plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Sus scrofa (Pig)) | BDBM50120841
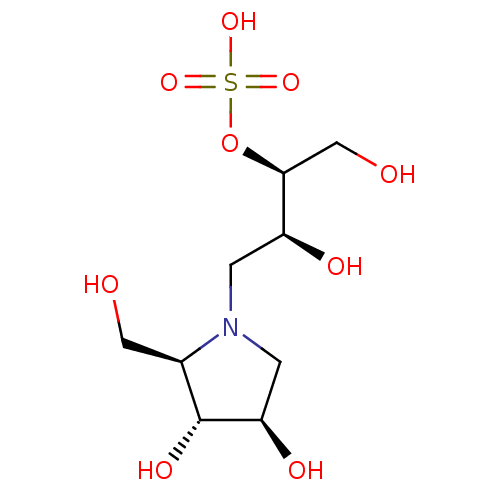
(CHEMBL3616593)Show SMILES OC[C@H](OS(O)(=O)=O)[C@@H](O)CN1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C9H19NO9S/c11-3-5-9(15)7(14)2-10(5)1-6(13)8(4-12)19-20(16,17)18/h5-9,11-15H,1-4H2,(H,16,17,18)/t5-,6+,7-,8+,9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of porcine pancreatic alpha-amylase using DP17 amylose as substrate preincubated for 5 mins followed by substrate addition by copper-bicin... |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Alpha-amylase type A isozyme
(Hordeum vulgare) | BDBM50120841

(CHEMBL3616593)Show SMILES OC[C@H](OS(O)(=O)=O)[C@@H](O)CN1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C9H19NO9S/c11-3-5-9(15)7(14)2-10(5)1-6(13)8(4-12)19-20(16,17)18/h5-9,11-15H,1-4H2,(H,16,17,18)/t5-,6+,7-,8+,9-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of recombinant barley alpha-amylase isozyme-1 using DP17 amylose as substrate preincubated for 5 mins followed by substrate addition by co... |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Pancreatic alpha-amylase
(Homo sapiens (Human)) | BDBM50120840

(CHEMBL1213470)Show SMILES OC[C@H]1N\C(=N\O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C6H12N2O5/c9-1-2-3(10)4(11)5(12)6(7-2)8-13/h2-5,9-13H,1H2,(H,7,8)/t2-,3-,4+,5-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Inhibition of human pancreatic alpha-amylase assessed as hydrolysis of G3F by Dixon plot analysis |
Bioorg Med Chem 23: 6725-32 (2015)
Article DOI: 10.1016/j.bmc.2015.09.007
BindingDB Entry DOI: 10.7270/Q21G0P2X |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572169
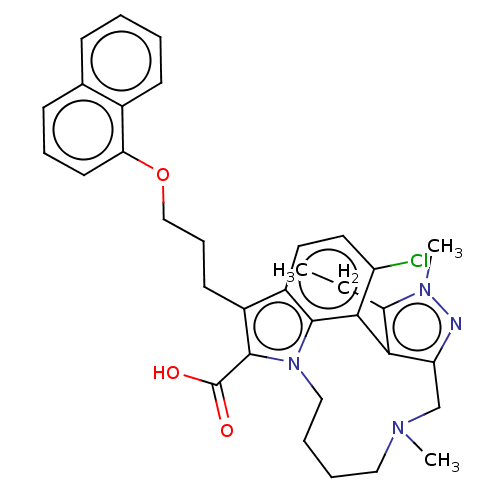
((rac)-4-chloro-3-ethyl-2,14-dimethyl-7-[3-(naphtha...)Show SMILES CCc1c-2c(CN(C)CCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572155

((rac)-4-chloro-3-ethyl-1-[2-(morpholin-4-yl)ethyl]...)Show SMILES Cc1nn(CCN2CCOCC2)c2COCCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c(-c12)c34 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572162

((+)-4-chloro-7-{3-[(6-fluoronaphthalen-1-yl)oxy]pr...)Show SMILES Cc1[nH]nc2COCCCCn3c(C(O)=O)c(CCCOc4cccc5cc(F)ccc45)c4ccc(Cl)c(-c12)c34 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572159

((+)-4-chloro-2,3,14-trimethyl-7-[3-(naphthalen-1-y...)Show SMILES CN1CCCCn2c(C(O)=O)c(CCCOc3cccc4ccccc34)c3ccc(Cl)c(-c4c(C)n(C)nc4C1)c23 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572181
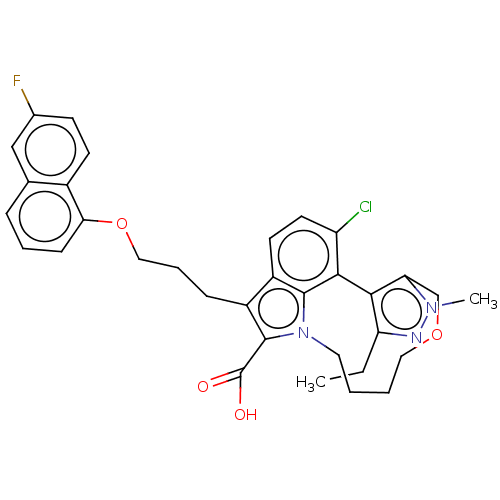
((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...)Show SMILES CCc1nn(C)c2COCCCCn3c(C(O)=O)c(CCCOc4cccc5cc(F)ccc45)c4ccc(Cl)c(-c12)c34 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572166
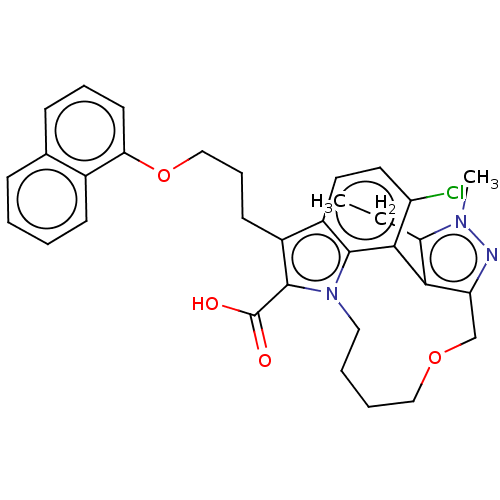
((rac)-4-chloro-3-ethyl-2-methyl-7-[3-(naphthalen-1...)Show SMILES CCc1c-2c(COCCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572152
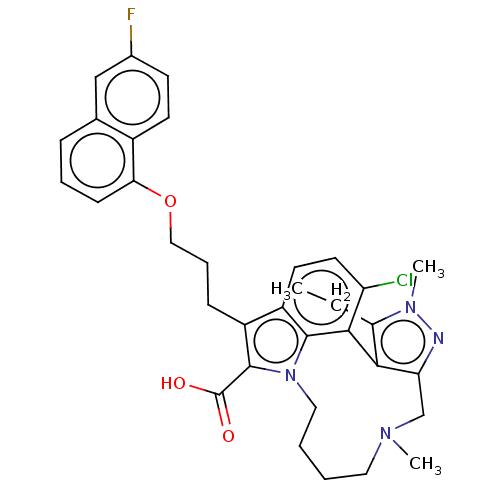
((rac)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1...)Show SMILES CCc1c-2c(CN(C)CCCCn3c(C(O)=O)c(CCCOc4cccc5cc(F)ccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572161
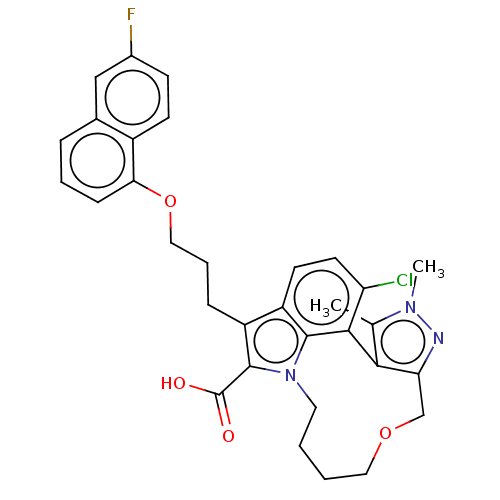
((rac)-4-chloro-7-{3-[(6-fluoronaphthalen-1-yl)oxy]...)Show SMILES Cc1c-2c(COCCCCn3c(C(O)=O)c(CCCOc4cccc5cc(F)ccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM625246

(2-(4-fluorophenyl)-N-[4-(4-oxo-3-phenyl-4,5,6,7-te...)Show SMILES Fc1ccc(CC(=O)Nc2cc(ccn2)-c2[nH]c3CCNC(=O)c3c2-c2ccccc2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572173
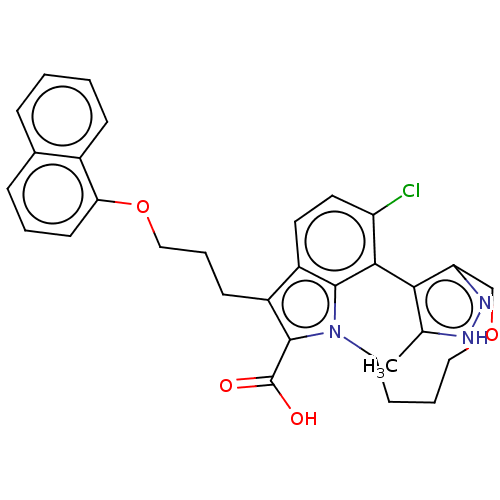
(4-chloro-2,3-dimethyl-7-[3-(naphthalen-1-yloxy)pro...)Show SMILES Cc1[nH]nc2COCCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c(-c12)c34 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572219
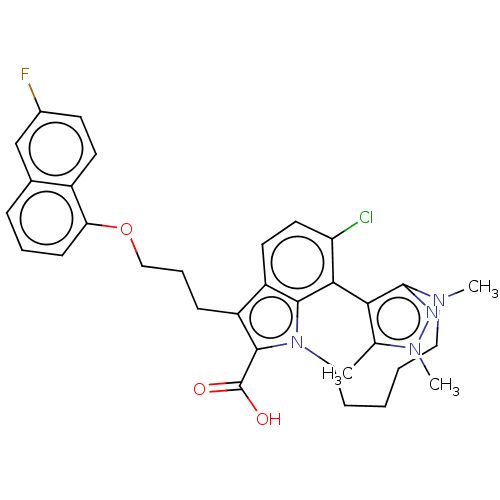
((rac)-4-chloro-7-{3-[(6-fluoronaphthalen-1-yl)oxy]...)Show SMILES CN1CCCCCn2c(C(O)=O)c(CCCOc3cccc4cc(F)ccc34)c3ccc(Cl)c(-c4c(C)n(C)nc14)c23 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572192
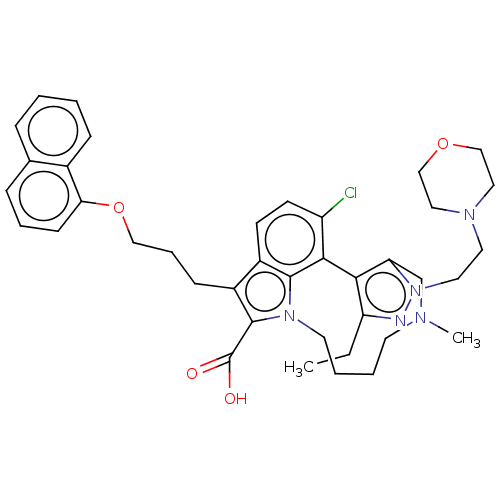
((rac)-4-chloro-3-ethyl-14-methyl-1-[2-(morpholin-4...)Show SMILES CCc1nn(CCN2CCOCC2)c2CN(C)CCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c(-c12)c34 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM625275
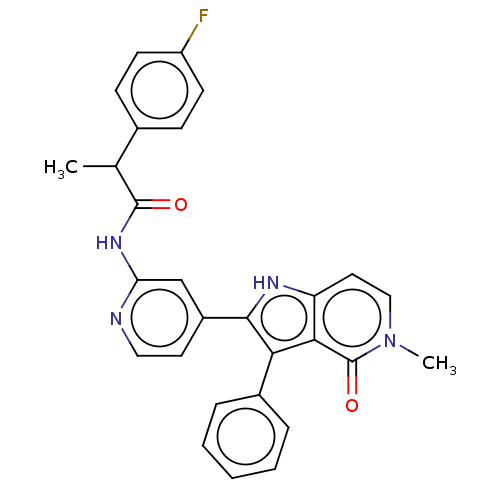
(2-(4-fluorophenyl)-N-[4-(5-methyl-4-oxo-3-phenyl-4...)Show SMILES CC(C(=O)Nc1cc(ccn1)-c1[nH]c2ccn(C)c(=O)c2c1-c1ccccc1)c1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
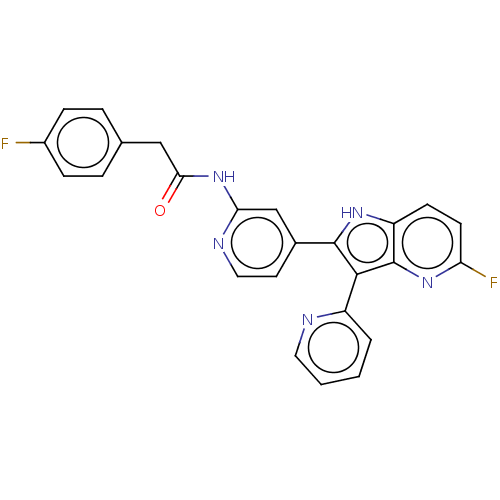
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572178
((+)-4-chloro-7-{3-[(6-fluoronaphthalen-1-yl)oxy]pr...)Show SMILES CN1CCCCn2c(C(O)=O)c(CCCOc3cccc4cc(F)ccc34)c3ccc(Cl)c(-c4c(C)[nH]nc4C1)c23 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
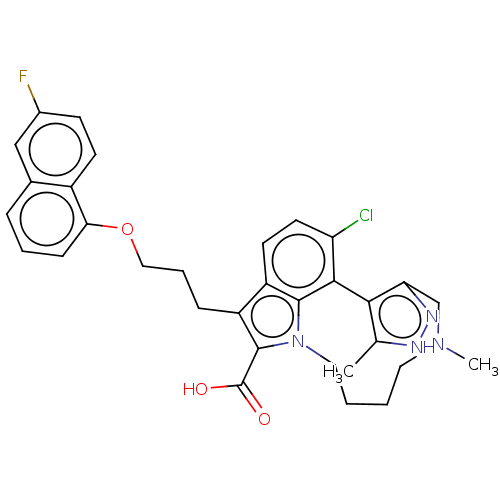
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM629242
((−)-N-{4-[5-fluoro-6-methyl-3-(pyridin-2-yl)...)Show SMILES Fc1ccc(CC(=O)Nc2cc(ccn2)-c2[nH]c3ccc(F)nc3c2-c2ccccn2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.739 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572196
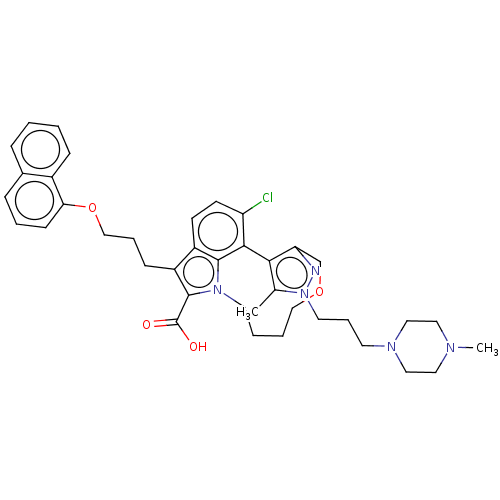
((+)-4-chloro-3-methyl-2-[3-(4-methylpiperazin-1-yl...)Show SMILES CN1CCN(CCCn2nc3COCCCCn4c(C(O)=O)c(CCCOc5cccc6ccccc56)c5ccc(Cl)c(-c3c2C)c45)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572184

((+)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1-y...)Show SMILES CCc1c-2c(COCCCCn3c(C(O)=O)c(CCCOc4cccc5cc(F)ccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572186

((rac)-4-chloro-3-ethyl-7-{3-[(6-fluoronaphthalen-1...)Show SMILES CCc1nn(C)c2CN(C)CCCCn3c(C(O)=O)c(CCCOc4cccc5cc(F)ccc45)c4ccc(Cl)c(-c12)c34 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572203
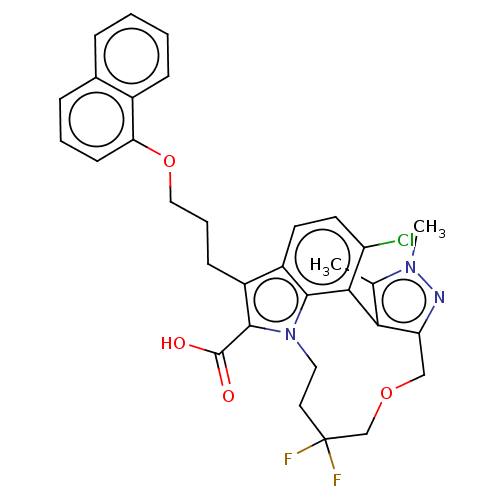
(4-chloro-12,12-difluoro-2,3-dimethyl-7-{3-[(naphth...)Show SMILES Cc1c-2c(COCC(F)(F)CCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572166

((rac)-4-chloro-3-ethyl-2-methyl-7-[3-(naphthalen-1...)Show SMILES CCc1c-2c(COCCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM625179
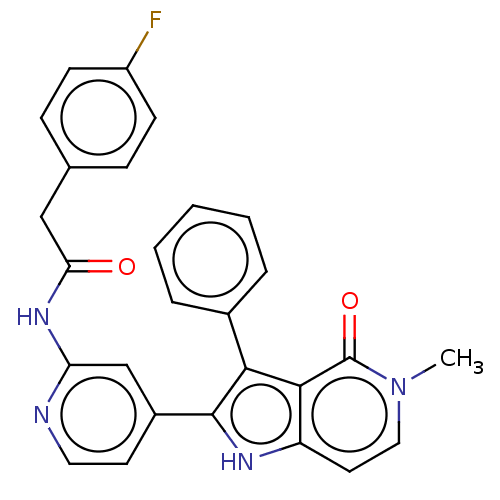
(2-(4-fluorophenyl)-N-[4-(5-methyl-4-oxo-3-phenyl-4...)Show SMILES Cn1ccc2[nH]c(c(-c3ccccc3)c2c1=O)-c1ccnc(NC(=O)Cc2ccc(F)cc2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572199
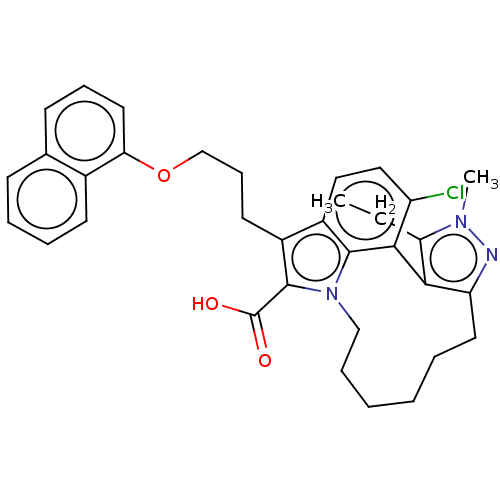
((+)-4-chloro-3-ethyl-2-methyl-7-{3-[(naphthalen-1-...)Show SMILES CCc1c-2c(CCCCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572155

((rac)-4-chloro-3-ethyl-1-[2-(morpholin-4-yl)ethyl]...)Show SMILES Cc1nn(CCN2CCOCC2)c2COCCCCn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c(-c12)c34 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572177
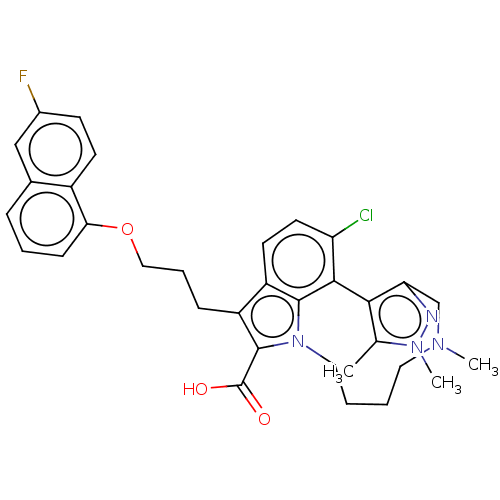
((rac)-4-chloro-7-{3-[(6-fluoronaphthalen-1-yl)oxy]...)Show SMILES CN1CCCCn2c(C(O)=O)c(CCCOc3cccc4cc(F)ccc34)c3ccc(Cl)c(-c4c(C)n(C)nc4C1)c23 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1 [173-321]/Noxa BH3 Peptide
(Homo sapiens (Human)) | BDBM572212
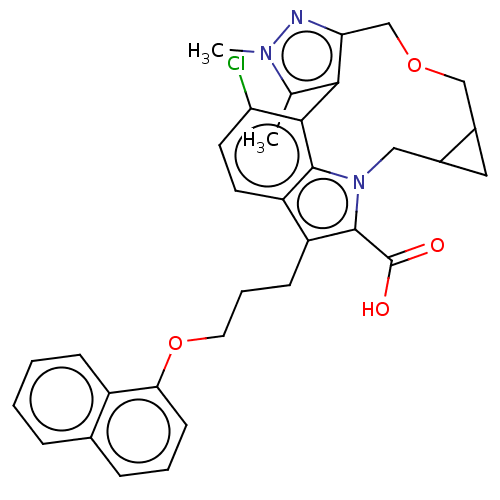
((9aS,10aR or 9aR, 10aS)-3-chloro-4,5-dimethyl-14-{...)Show SMILES Cc1c-2c(COCC3CC3Cn3c(C(O)=O)c(CCCOc4cccc5ccccc45)c4ccc(Cl)c-2c34)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The dose-dependent inhibition by the compounds described in this invention of the interaction between MCL-1 and the BH3 domain of Noxa (both human) w... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2057K58 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM625180
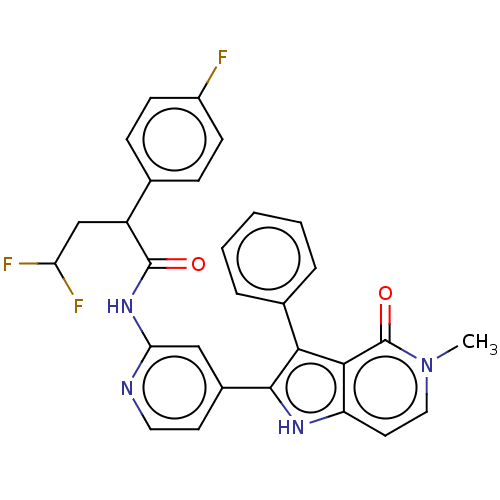
(4,4-difluoro-2-(4-fluorophenyl)-N-[4-(5-methyl-4-o...)Show SMILES Cn1ccc2[nH]c(c(-c3ccccc3)c2c1=O)-c1ccnc(NC(=O)C(CC(F)F)c2ccc(F)cc2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data