| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase |
|---|
| Ligand | BDBM50090510 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1498284 (CHEMBL3583805) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Sato, K; Takahagi, H; Yoshikawa, T; Morimoto, S; Takai, T; Hidaka, K; Kamaura, M; Kubo, O; Adachi, R; Ishii, T; Maki, T; Mochida, T; Takekawa, S; Nakakariya, M; Amano, N; Kitazaki, T Discovery of a Novel Series of N-Phenylindoline-5-sulfonamide Derivatives as Potent, Selective, and Orally Bioavailable Acyl CoA:Monoacylglycerol Acyltransferase-2 Inhibitors. J Med Chem58:3892-909 (2015) [PubMed] Article Sato, K; Takahagi, H; Yoshikawa, T; Morimoto, S; Takai, T; Hidaka, K; Kamaura, M; Kubo, O; Adachi, R; Ishii, T; Maki, T; Mochida, T; Takekawa, S; Nakakariya, M; Amano, N; Kitazaki, T Discovery of a Novel Series of N-Phenylindoline-5-sulfonamide Derivatives as Potent, Selective, and Orally Bioavailable Acyl CoA:Monoacylglycerol Acyltransferase-2 Inhibitors. J Med Chem58:3892-909 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase |
|---|
| Name: | Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase |
|---|
| Synonyms: | GGNT3 | GNT-III | GlcNAc-T III | MGAT3 | MGAT3_HUMAN | N-acetylglucosaminyltransferase III | N-glycosyl-oligosaccharide-glycoprotein N-acetylglucosaminyltransferase III |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 61324.81 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_107586 |
|---|
| Residue: | 533 |
|---|
| Sequence: | MKMRRYKLFLMFCMAGLCLISFLHFFKTLSYVTFPRELASLSPNLVSSFFWNNAPVTPQA
SPEPGGPDLLRTPLYSHSPLLQPLPPSKAAEELHRVDLVLPEDTTEYFVRTKAGGVCFKP
GTKMLERPPPGRPEEKPEGANGSSARRPPRYLLSARERTGGRGARRKWVECVCLPGWHGP
SCGVPTVVQYSNLPTKERLVPREVPRRVINAINVNHEFDLLDVRFHELGDVVDAFVVCES
NFTAYGEPRPLKFREMLTNGTFEYIRHKVLYVFLDHFPPGGRQDGWIADDYLRTFLTQDG
VSRLRNLRPDDVFIIDDADEIPARDGVLFLKLYDGWTEPFAFHMRKSLYGFFWKQPGTLE
VVSGCTVDMLQAVYGLDGIRLRRRQYYTMPNFRQYENRTGHILVQWSLGSPLHFAGWHCS
WCFTPEGIYFKLVSAQNGDFPRWGDYEDKRDLNYIRGLIRTGGWFDGTQQEYPPADPSEH
MYAPKYLLKNYDRFHYLLDNPYQEPRSTAAGGWRHRGPEGRPPARGKLDEAEV
|
|
|
|---|
| BDBM50090510 |
|---|
| n/a |
|---|
| Name | BDBM50090510 |
|---|
| Synonyms: | CHEMBL3581716 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H23F2N3O3S |
|---|
| Mol. Mass. | 471.52 |
|---|
| SMILES | CC(=O)Nc1cc(cc2CCN(CCc3ccccc3)c12)S(=O)(=O)Nc1ccc(F)cc1F |
|---|
| Structure | 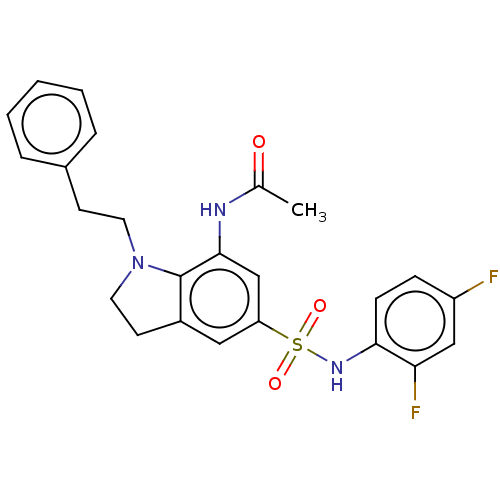
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sato, K; Takahagi, H; Yoshikawa, T; Morimoto, S; Takai, T; Hidaka, K; Kamaura, M; Kubo, O; Adachi, R; Ishii, T; Maki, T; Mochida, T; Takekawa, S; Nakakariya, M; Amano, N; Kitazaki, T Discovery of a Novel Series of N-Phenylindoline-5-sulfonamide Derivatives as Potent, Selective, and Orally Bioavailable Acyl CoA:Monoacylglycerol Acyltransferase-2 Inhibitors. J Med Chem58:3892-909 (2015) [PubMed] Article
Sato, K; Takahagi, H; Yoshikawa, T; Morimoto, S; Takai, T; Hidaka, K; Kamaura, M; Kubo, O; Adachi, R; Ishii, T; Maki, T; Mochida, T; Takekawa, S; Nakakariya, M; Amano, N; Kitazaki, T Discovery of a Novel Series of N-Phenylindoline-5-sulfonamide Derivatives as Potent, Selective, and Orally Bioavailable Acyl CoA:Monoacylglycerol Acyltransferase-2 Inhibitors. J Med Chem58:3892-909 (2015) [PubMed] Article