| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50169698 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1576886 (CHEMBL3806595) |
|---|
| IC50 | >25000±n/a nM |
|---|
| Citation |  Kavanagh, ME; Coyne, AG; McLean, KJ; James, GG; Levy, CW; Marino, LB; de Carvalho, LP; Chan, DS; Hudson, SA; Surade, S; Leys, D; Munro, AW; Abell, C Fragment-Based Approaches to the Development of Mycobacterium tuberculosis CYP121 Inhibitors. J Med Chem59:3272-302 (2016) [PubMed] Article Kavanagh, ME; Coyne, AG; McLean, KJ; James, GG; Levy, CW; Marino, LB; de Carvalho, LP; Chan, DS; Hudson, SA; Surade, S; Leys, D; Munro, AW; Abell, C Fragment-Based Approaches to the Development of Mycobacterium tuberculosis CYP121 Inhibitors. J Med Chem59:3272-302 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50169698 |
|---|
| n/a |
|---|
| Name | BDBM50169698 |
|---|
| Synonyms: | CHEMBL3806191 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H14N4 |
|---|
| Mol. Mass. | 250.2985 |
|---|
| SMILES | Nc1[nH]ncc1-c1cccc(c1)-c1cccc(N)c1 |
|---|
| Structure | 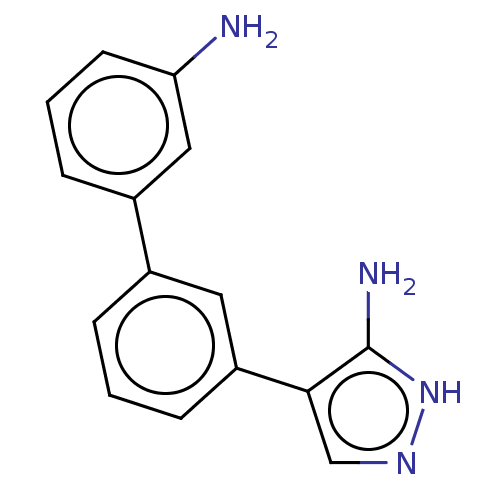
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kavanagh, ME; Coyne, AG; McLean, KJ; James, GG; Levy, CW; Marino, LB; de Carvalho, LP; Chan, DS; Hudson, SA; Surade, S; Leys, D; Munro, AW; Abell, C Fragment-Based Approaches to the Development of Mycobacterium tuberculosis CYP121 Inhibitors. J Med Chem59:3272-302 (2016) [PubMed] Article
Kavanagh, ME; Coyne, AG; McLean, KJ; James, GG; Levy, CW; Marino, LB; de Carvalho, LP; Chan, DS; Hudson, SA; Surade, S; Leys, D; Munro, AW; Abell, C Fragment-Based Approaches to the Development of Mycobacterium tuberculosis CYP121 Inhibitors. J Med Chem59:3272-302 (2016) [PubMed] Article