| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Proteasome subunit beta type-2 |
|---|
| Ligand | BDBM50069985 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1618121 (CHEMBL3860290) |
|---|
| Ki | 22100±n/a nM |
|---|
| Citation |  Di Giovanni, C; Ettari, R; Sarno, S; Rotondo, A; Bitto, A; Squadrito, F; Altavilla, D; Schirmeister, T; Novellino, E; Grasso, S; Zappalą, M; Lavecchia, A Identification of noncovalent proteasome inhibitors with high selectivity for chymotrypsin-like activity by a multistep structure-based virtual screening. Eur J Med Chem121:578-591 (2016) [PubMed] Article Di Giovanni, C; Ettari, R; Sarno, S; Rotondo, A; Bitto, A; Squadrito, F; Altavilla, D; Schirmeister, T; Novellino, E; Grasso, S; Zappalą, M; Lavecchia, A Identification of noncovalent proteasome inhibitors with high selectivity for chymotrypsin-like activity by a multistep structure-based virtual screening. Eur J Med Chem121:578-591 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Proteasome subunit beta type-2 |
|---|
| Name: | Proteasome subunit beta type-2 |
|---|
| Synonyms: | 20S proteasome | PSB2_HUMAN | PSMB2 | Proteasome Macropain subunit |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 22837.53 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1294233 |
|---|
| Residue: | 201 |
|---|
| Sequence: | MEYLIGIQGPDYVLVASDRVAASNIVQMKDDHDKMFKMSEKILLLCVGEAGDTVQFAEYI
QKNVQLYKMRNGYELSPTAAANFTRRNLADCLRSRTPYHVNLLLAGYDEHEGPALYYMDY
LAALAKAPFAAHGYGAFLTLSILDRYYTPTISRERAVELLRKCLEELQKRFILNLPTFSV
RIIDKNGIHDLDNISFPKQGS
|
|
|
|---|
| BDBM50069985 |
|---|
| n/a |
|---|
| Name | BDBM50069985 |
|---|
| Synonyms: | (S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic acid [(S)-1-((S)-1-formyl-3-methyl-butylcarbamoyl)-3-methyl-butyl]-amide | CHEMBL64925 | Cbz-L-leu-L-leu-L-leu-CHO | MG-13 | MG-132 | Z-L-leu-L-leu-L-leu-H | Z-Leu-Leu-Leu-H | Z-Leu-Leu-Leu-al | benzyl (S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate | benzyl(S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate | benzyloxycarbonyl-Leu-Leu-leucinal | {(S)-1-[(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester | {1-[(S)-(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester | {1-[1-(1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H41N3O5 |
|---|
| Mol. Mass. | 475.6208 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| |
|---|
| Structure | 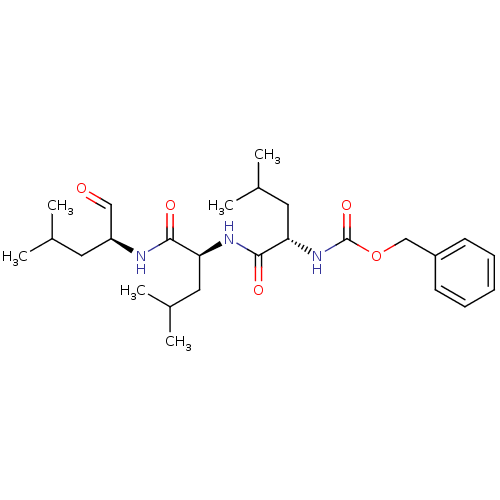
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Di Giovanni, C; Ettari, R; Sarno, S; Rotondo, A; Bitto, A; Squadrito, F; Altavilla, D; Schirmeister, T; Novellino, E; Grasso, S; Zappalą, M; Lavecchia, A Identification of noncovalent proteasome inhibitors with high selectivity for chymotrypsin-like activity by a multistep structure-based virtual screening. Eur J Med Chem121:578-591 (2016) [PubMed] Article
Di Giovanni, C; Ettari, R; Sarno, S; Rotondo, A; Bitto, A; Squadrito, F; Altavilla, D; Schirmeister, T; Novellino, E; Grasso, S; Zappalą, M; Lavecchia, A Identification of noncovalent proteasome inhibitors with high selectivity for chymotrypsin-like activity by a multistep structure-based virtual screening. Eur J Med Chem121:578-591 (2016) [PubMed] Article