| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Ligand | BDBM50221512 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_208265 (CHEMBL813225) |
|---|
| IC50 | 63.1±n/a nM |
|---|
| Citation |  Hotoda, H; Daigo, M; Furukawa, M; Murayama, K; Hasegawa, CA; Kaneko, M; Muramatsu, Y; Ishii, MM; Miyakoshi, S; Takatsu, T; Inukai, M; Kakuta, M; Abe, T; Fukuoka, T; Utsui, Y; Ohya, S Synthesis and antimycobacterial activity of capuramycin analogues. Part 2: acylated derivatives of capuramycin-related compounds. Bioorg Med Chem Lett13:2833-6 (2003) [PubMed] Hotoda, H; Daigo, M; Furukawa, M; Murayama, K; Hasegawa, CA; Kaneko, M; Muramatsu, Y; Ishii, MM; Miyakoshi, S; Takatsu, T; Inukai, M; Kakuta, M; Abe, T; Fukuoka, T; Utsui, Y; Ohya, S Synthesis and antimycobacterial activity of capuramycin analogues. Part 2: acylated derivatives of capuramycin-related compounds. Bioorg Med Chem Lett13:2833-6 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Name: | Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Synonyms: | MRAY_ECOLI | Phospho-N-acetylmuramoyl-pentapeptide-transferase/UDP-N-acetylglucosamine--N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase | mraY | murX |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 39889.38 |
|---|
| Organism: | Escherichia coli (strain K12) |
|---|
| Description: | ChEMBL_1454117 |
|---|
| Residue: | 360 |
|---|
| Sequence: | MLVWLAEHLVKYYSGFNVFSYLTFRAIVSLLTALFISLWMGPRMIAHLQKLSFGQVVRND
GPESHFSKRGTPTMGGIMILTAIVISVLLWAYPSNPYVWCVLVVLVGYGVIGFVDDYRKV
VRKDTKGLIARWKYFWMSVIALGVAFALYLAGKDTPATQLVVPFFKDVMPQLGLFYILLA
YFVIVGTGNAVNLTDGLDGLAIMPTVFVAGGFALVAWATGNMNFASYLHIPYLRHAGELV
IVCTAIVGAGLGFLWFNTYPAQVFMGDVGSLALGGALGIIAVLLRQEFLLVIMGGVFVVE
TLSVILQVGSFKLRGQRIFRMAPIHHHYELKGWPEPRVIVRFWIISLMLVLIGLATLKVR
|
|
|
|---|
| BDBM50221512 |
|---|
| n/a |
|---|
| Name | BDBM50221512 |
|---|
| Synonyms: | CHEMBL318366 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H43N5O13 |
|---|
| Mol. Mass. | 681.6881 |
|---|
| SMILES | [H][C@@]1(O[C@H]([C@H](OC(=O)CCCCC)[C@@H]1OC)n1ccc(=O)[nH]c1=O)[C@@H](O[C@H]1OC(=C[C@H](O)[C@@H]1O)C(=O)N[C@H]1CCC[C@@H](C)NC1=O)C(N)=O |c:30| |
|---|
| Structure | 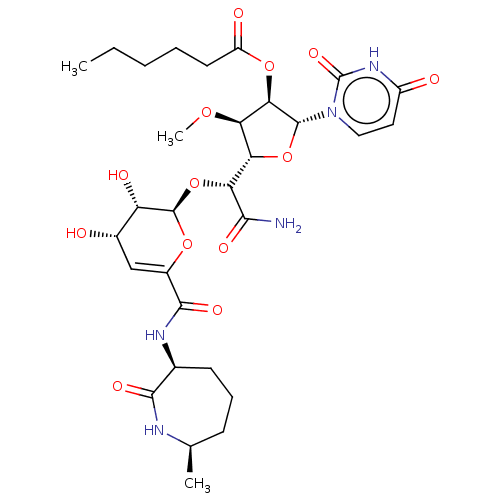
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hotoda, H; Daigo, M; Furukawa, M; Murayama, K; Hasegawa, CA; Kaneko, M; Muramatsu, Y; Ishii, MM; Miyakoshi, S; Takatsu, T; Inukai, M; Kakuta, M; Abe, T; Fukuoka, T; Utsui, Y; Ohya, S Synthesis and antimycobacterial activity of capuramycin analogues. Part 2: acylated derivatives of capuramycin-related compounds. Bioorg Med Chem Lett13:2833-6 (2003) [PubMed]
Hotoda, H; Daigo, M; Furukawa, M; Murayama, K; Hasegawa, CA; Kaneko, M; Muramatsu, Y; Ishii, MM; Miyakoshi, S; Takatsu, T; Inukai, M; Kakuta, M; Abe, T; Fukuoka, T; Utsui, Y; Ohya, S Synthesis and antimycobacterial activity of capuramycin analogues. Part 2: acylated derivatives of capuramycin-related compounds. Bioorg Med Chem Lett13:2833-6 (2003) [PubMed]