| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 3A |
|---|
| Ligand | BDBM50222218 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_3109 (CHEMBL620587) |
|---|
| Ki | 252±n/a nM |
|---|
| Citation |  Rosen, T; Seeger, TF; McLean, S; Nagel, AA; Ives, JL; Guarino, KJ; Bryce, D; Furman, J; Roth, RW; Chalabi, PM Synthesis, in vitro binding profile, and central nervous system penetrability of the highly potent 5-HT3 receptor antagonist [3H]-4-(2-methoxyphenyl)-2-[4(5)-methyl-5(4)-imidazolylmethyl]thiazole. J Med Chem33:3020-3 (1990) [PubMed] Rosen, T; Seeger, TF; McLean, S; Nagel, AA; Ives, JL; Guarino, KJ; Bryce, D; Furman, J; Roth, RW; Chalabi, PM Synthesis, in vitro binding profile, and central nervous system penetrability of the highly potent 5-HT3 receptor antagonist [3H]-4-(2-methoxyphenyl)-2-[4(5)-methyl-5(4)-imidazolylmethyl]thiazole. J Med Chem33:3020-3 (1990) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 3A |
|---|
| Name: | 5-hydroxytryptamine receptor 3A |
|---|
| Synonyms: | 5-HT-3 | 5-HT3-A | 5-HT3A | 5-HT3A Serotonin Receptor | 5-HT3R | 5-hydroxytryptamine receptor 3 | 5-hydroxytryptamine receptor 3A | 5HT3A_MOUSE | 5ht3 | Htr3 | Htr3a | Serotonin 3 receptor (5HT3) | Serotonin 3a (5-HT3a) receptor | Serotonin receptor 3A | Serotonin-gated ion channel receptor |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 56056.00 |
|---|
| Organism: | Mus musculus (house mouse) |
|---|
| Description: | 5HT3A |
|---|
| Residue: | 487 |
|---|
| Sequence: | MRLCIPQVLLALFLSMLTAPGEGSRRRATQEDTTQPALLRLSDHLLANYKKGVRPVRDWR
KPTTVSIDVIMYAILNVDEKNQVLTTYIWYRQYWTDEFLQWTPEDFDNVTKLSIPTDSIW
VPDILINEFVDVGKSPNIPYVYVHHRGEVQNYKPLQLVTACSLDIYNFPFDVQNCSLTFT
SWLHTIQDINITLWRSPEEVRSDKSIFINQGEWELLEVFPQFKEFSIDISNSYAEMKFYV
IIRRRPLFYAVSLLLPSIFLMVVDIVGFCLPPDSGERVSFKITLLLGYSVFLIIVSDTLP
ATIGTPLIGVYFVVCMALLVISLAETIFIVRLVHKQDLQRPVPDWLRHLVLDRIAWILCL
GEQPMAHRPPATFQANKTDDCSGSDLLPAMGNHCSHVGGPQDLEKTPRGRGSPLPPPREA
SLAVRGLLQELSSIRHFLEKRDEMREVARDWLRVGYVLDRLLFRIYLLAVLAYSITLVTL
WSIWHYS
|
|
|
|---|
| BDBM50222218 |
|---|
| n/a |
|---|
| Name | BDBM50222218 |
|---|
| Synonyms: | CHEBI:51137 | Mianserin |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H20N2 |
|---|
| Mol. Mass. | 264.3648 |
|---|
| SMILES | CN1CCN2C(C1)c1ccccc1Cc1ccccc21 |
|---|
| Structure | 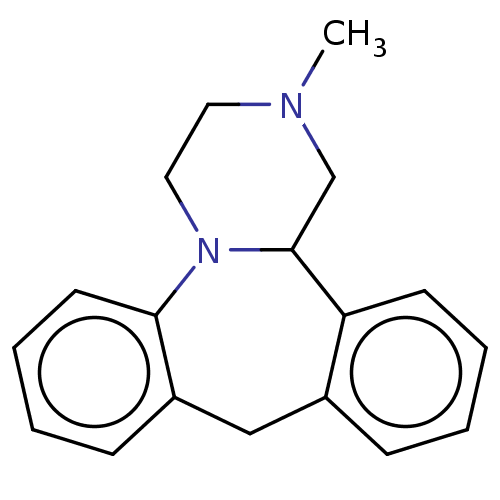
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Rosen, T; Seeger, TF; McLean, S; Nagel, AA; Ives, JL; Guarino, KJ; Bryce, D; Furman, J; Roth, RW; Chalabi, PM Synthesis, in vitro binding profile, and central nervous system penetrability of the highly potent 5-HT3 receptor antagonist [3H]-4-(2-methoxyphenyl)-2-[4(5)-methyl-5(4)-imidazolylmethyl]thiazole. J Med Chem33:3020-3 (1990) [PubMed]
Rosen, T; Seeger, TF; McLean, S; Nagel, AA; Ives, JL; Guarino, KJ; Bryce, D; Furman, J; Roth, RW; Chalabi, PM Synthesis, in vitro binding profile, and central nervous system penetrability of the highly potent 5-HT3 receptor antagonist [3H]-4-(2-methoxyphenyl)-2-[4(5)-methyl-5(4)-imidazolylmethyl]thiazole. J Med Chem33:3020-3 (1990) [PubMed]