| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5'-nucleotidase |
|---|
| Ligand | BDBM222261 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Alkaline Phosphatase Assay |
|---|
| pH | 9.8±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| IC50 | 3.3e+2± 4 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Ejaz, SA; Saeed, A; Siddique, MN; Nisa, ZU; Khan, S; Lecka, J; Sévigny, J; Iqbal, J Synthesis, characterization and biological evaluation of novel chalcone sulfonamide hybrids as potent intestinal alkaline phosphatase inhibitors. Bioorg Chem70:229-236 (2017) [PubMed] Article Ejaz, SA; Saeed, A; Siddique, MN; Nisa, ZU; Khan, S; Lecka, J; Sévigny, J; Iqbal, J Synthesis, characterization and biological evaluation of novel chalcone sulfonamide hybrids as potent intestinal alkaline phosphatase inhibitors. Bioorg Chem70:229-236 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5'-nucleotidase |
|---|
| Name: | 5'-nucleotidase |
|---|
| Synonyms: | 5'-nucleotidase | 5NTD_RAT | Ecto-5'-nucleotidase (e5'NT) | Ecto-5-nucleotidase (e5'NT) | NT | Nt5 | Nt5e | Nte |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 63971.44 |
|---|
| Organism: | Rattus norvegicus (Rat) |
|---|
| Description: | P21588 |
|---|
| Residue: | 576 |
|---|
| Sequence: | MRPAAATAPKWLLLALSALLPLWPTAKSWELTIMHTNDVHSRLEQTSDDSTKCLNASLCV
GGVARLFTKVQQIRKEEPNVLLLDAGDQYQGTIWFTVYKGLEVAHFMNLLGYDAMALGNH
EFDNGVEGLIDPLLRNVKFPILSANIKARGPLAPQISGLYLPYKVLSVGGEVVGIVGYTS
KETPFLSNPGTNLVFEDEVTALQPEVDKLKTLNVNKIIALGHSGFEMDKLIAQKVRGVDV
VVGGHTNTFLYTGNPPSKEVPAGKYPFIVTSDDGRKVPVVQAYAFGKYLGYLKVEFDDKG
NVVTSYGNPILLNSTIREDAAIKADINQWRIKLDNYSTQELGRTIVYLNGSAQECRFREC
NMGNLICDAMINNNLRHPDEMFWNHVSMCIVNGGGIRSPIDERNNGTITWENLAAVLPFG
GTFDLVQLKGSTLKKAFEHSVHRYGQSTGEFLQVGGIHVVYDISRKPWDRVVQLKVLCTK
CRVPIYEPLEMDKVYKVVLPSYLVNGGDGFQMIKDELLKHDSGDQDISVVSEYISKMKVI
YPAVEGRIKFSAASHYQGSFPLIILSFWAVILVLYQ
|
|
|
|---|
| BDBM222261 |
|---|
| n/a |
|---|
| Name | BDBM222261 |
|---|
| Synonyms: | (Z)-4-(2-((E)-4-(4-methoxyphenyl)-1-morpholino-2-oxobut-3-enylidene)hydrazinyl) benzenesulfonamide (4d) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H24N4O5S |
|---|
| Mol. Mass. | 444.504 |
|---|
| SMILES | COc1ccc(\C=C\C(=O)C(=N\Nc2ccc(cc2)S(N)(=O)=O)\N2CCOCC2)cc1 |
|---|
| Structure | 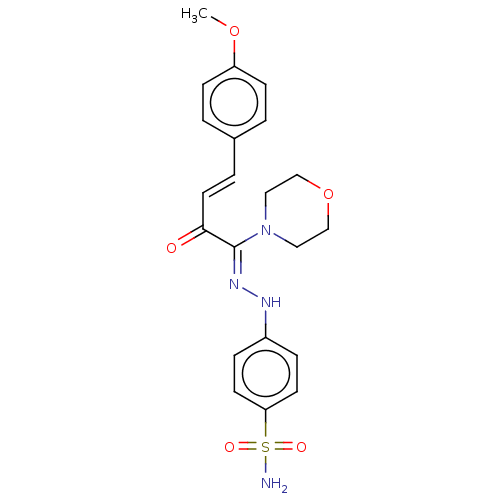
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ejaz, SA; Saeed, A; Siddique, MN; Nisa, ZU; Khan, S; Lecka, J; Sévigny, J; Iqbal, J Synthesis, characterization and biological evaluation of novel chalcone sulfonamide hybrids as potent intestinal alkaline phosphatase inhibitors. Bioorg Chem70:229-236 (2017) [PubMed] Article
Ejaz, SA; Saeed, A; Siddique, MN; Nisa, ZU; Khan, S; Lecka, J; Sévigny, J; Iqbal, J Synthesis, characterization and biological evaluation of novel chalcone sulfonamide hybrids as potent intestinal alkaline phosphatase inhibitors. Bioorg Chem70:229-236 (2017) [PubMed] Article