

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Poly [ADP-ribose] polymerase tankyrase-1 [1091-1325] | ||
| Ligand | BDBM50444583 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Tankyrase Biochemical Assays | ||
| Temperature | 298.15±n/a K | ||
| IC50 | 1.47±n/a nM | ||
| Comments | extracted | ||
| Citation |  Bregman, H; Buchanan, JL; Chakka, N; Dimauro, EF; Gunaydin, H; Guzman-Perez, A; Hua, Z; Huang, H; Huang, X; Martin, MW; Patel, VF Oxazolidinone compounds and derivatives thereof US Patent US9340549 Publication Date 5/17/2016 Bregman, H; Buchanan, JL; Chakka, N; Dimauro, EF; Gunaydin, H; Guzman-Perez, A; Hua, Z; Huang, H; Huang, X; Martin, MW; Patel, VF Oxazolidinone compounds and derivatives thereof US Patent US9340549 Publication Date 5/17/2016 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Poly [ADP-ribose] polymerase tankyrase-1 [1091-1325] | |||
| Name: | Poly [ADP-ribose] polymerase tankyrase-1 [1091-1325] | ||
| Synonyms: | PARP5A | PARPL | TIN1 | TINF1 | TNKS | TNKS1 | TNKS1_HUMAN | Tankyrase-1 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 26714.90 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | O95271[1091-1325] | ||
| Residue: | 235 | ||
| Sequence: |
| ||
| BDBM50444583 | |||
| n/a | |||
| Name | BDBM50444583 | ||
| Synonyms: | CHEMBL3099716 | US9340549, 74 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C26H29N5O2 | ||
| Mol. Mass. | 443.5408 | ||
| SMILES | CC1(C)OC(=O)N([C@H]1c1ccccc1)[C@H]1CC[C@@H](CC1)c1cnc(N)c(c1)-c1ncccn1 |r,wU:17.22,7.8,wD:14.15,(49.17,-3.5,;50.51,-4.26,;49.19,-5.04,;51.4,-3,;52.87,-3.46,;54.11,-2.55,;52.89,-5,;51.43,-5.5,;50.7,-6.85,;49.16,-6.89,;48.43,-8.25,;49.24,-9.56,;50.79,-9.51,;51.51,-8.15,;54.14,-5.9,;54.14,-7.45,;55.47,-8.22,;56.81,-7.45,;56.8,-5.9,;55.47,-5.13,;58.14,-8.21,;59.46,-7.44,;60.8,-8.2,;60.8,-9.75,;62.14,-10.52,;59.47,-10.52,;58.14,-9.76,;59.47,-12.05,;58.14,-12.83,;58.14,-14.36,;59.47,-15.13,;60.81,-14.35,;60.8,-12.82,)| | ||
| Structure | 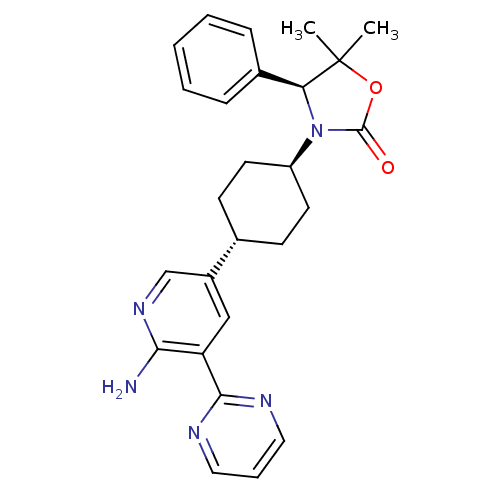
| ||